Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study
- PMID: 34011492
- PMCID: PMC8132065
- DOI: 10.1136/bmj.n1098
Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study
Abstract
Objective: To evaluate the excess risk and relative hazards for developing incident clinical sequelae after the acute phase of SARS-CoV-2 infection in adults aged 18-65.
Design: Retrospective cohort study.
Setting: Three merged data sources from a large United States health plan: a large national administrative claims database, an outpatient laboratory testing database, and an inpatient hospital admissions database.
Participants: Individuals aged 18-65 with continuous enrollment in the health plan from January 2019 to the date of a diagnosis of SARS-CoV-2 infection. Three comparator groups, matched by propensity score, to individuals infected with SARS-CoV-2: a 2020 comparator group, an historical 2019 comparator group, and an historical comparator group with viral lower respiratory tract illness.
Main outcome measures: More than 50 clinical sequelae after the acute phase of SARS-CoV-2 infection (defined as the date of first SARS-CoV-2 diagnosis (index date) plus 21 days) were identified using ICD-10 (international classification of diseases, 10th revision) codes. Excess risk in the four months after acute infection and hazard ratios with Bonferroni corrected 95% confidence intervals were calculated.
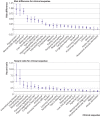
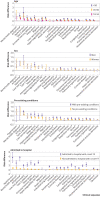
Results: 14% of adults aged ≤65 who were infected with SARS-CoV-2 (27 074 of 193 113) had at least one new type of clinical sequelae that required medical care after the acute phase of the illness, which was 4.95% higher than in the 2020 comparator group. The risk for specific new sequelae attributable to SARS-Cov-2 infection after the acute phase, including chronic respiratory failure, cardiac arrythmia, hypercoagulability, encephalopathy, peripheral neuropathy, amnesia (memory difficulty), diabetes, liver test abnormalities, myocarditis, anxiety, and fatigue, was significantly greater than in the three comparator groups (2020, 2019, and viral lower respiratory tract illness groups) (all P<0.001). Significant risk differences because of SARS-CoV-2 infection ranged from 0.02 to 2.26 per 100 people (all P<0.001), and hazard ratios ranged from 1.24 to 25.65 compared with the 2020 comparator group.
Conclusions: The results indicate the excess risk of developing new clinical sequelae after the acute phase of SARS-CoV-2 infection, including specific types of sequelae less commonly seen in other viral illnesses. Although individuals who were older, had pre-existing conditions, and were admitted to hospital because of covid-19 were at greatest excess risk, younger adults (aged ≤50), those with no pre-existing conditions, or those not admitted to hospital for covid-19 also had an increased risk of developing new clinical sequelae. The greater risk for incident sequelae after the acute phase of SARS-CoV-2 infection is relevant for healthcare planning.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from OptumLabs for the submitted work and no other organization or company provided support for this work; SED, YG, KH, JS, MCD, KGJ declare that they have had no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted. KC declares consulting from Pfizer. ML declares honoraria/consulting from Merck, Sanofi-Pasteur, Bristol Myers-Squibb, and Antigen Discovery; research funding (institutional) from Pfizer, and unpaid scientific advice to Janssen, Astra-Zeneca, One Day Sooner, and Covaxx (United Biomedical).
Figures

Comment in
-
Unpacking post-covid symptoms.BMJ. 2021 May 19;373:n1173. doi: 10.1136/bmj.n1173. BMJ. 2021. PMID: 34011496 No abstract available.
References
-
- Sudre CH, Murray B, Varsavsky T, et al. . Attributes and predictors of Long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. medRxiv [Preprint] 2020;2020.10.19.20214494.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
