Negative Regulation of Human Hepatic Constitutive Androstane Receptor by Cholesterol Synthesis Inhibition: Role of Sterol Regulatory Element Binding Proteins
- PMID: 34011532
- PMCID: PMC11025015
- DOI: 10.1124/dmd.120.000341
Negative Regulation of Human Hepatic Constitutive Androstane Receptor by Cholesterol Synthesis Inhibition: Role of Sterol Regulatory Element Binding Proteins
Abstract
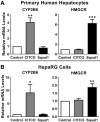
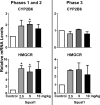
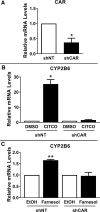
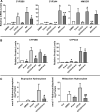
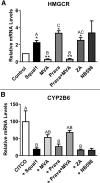
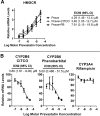
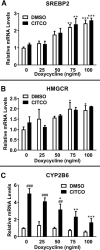
The squalene synthase inhibitor squalestatin 1 (Squal1) is a potent and efficacious inducer of CYP2B expression in primary cultured rat hepatocytes and rat liver. To determine whether Squal1 is also an inducer of human CYP2B, the effects of Squal1 treatment were evaluated in primary cultured human hepatocytes, differentiated HepaRG cells, and humanized mouse livers. Squal1 treatment did not increase CYP2B6 mRNA levels in human hepatocytes or HepaRG cells and only slightly and inconsistently increased CYP2B6 mRNA content in humanized mouse liver. However, treatment with farnesol, which mediates Squal1's effect on rat CYP2B expression, increased CYP2B6 mRNA levels in HepaRG cells expressing the constitutive androstane receptor (CAR), but not in cells with knocked-down CAR. To determine the impact of cholesterol biosynthesis inhibition on CAR activation, the effects of pravastatin (Prava) were determined on CITCO-mediated gene expression in primary cultured human hepatocytes. Prava treatment abolished CITCO-inducible CYP2B6 expression, but had less effect on rifampicin-mediated CYP3A4 induction, and CITCO treatment did not affect Prava-inducible HMG-CoA reductase (HMGCR) expression. Treatment with inhibitors of different steps of cholesterol biosynthesis attenuated CITCO-mediated CYP2B6 induction in HepaRG cells, and Prava treatment increased HMGCR expression and inhibited CYP2B6 induction with comparable potency. Transfection of HepG2 cells with transcriptionally active sterol regulatory element binding proteins (SREBPs) reduced CAR-mediated transactivation, and inducible expression of transcriptionally active SREBP2 attenuated CITCO-inducible CYP2B6 expression in HepaRG cells. These findings suggest that Squal1 does not induce CYP2B6 in human hepatocytes because Squal1's inhibitory effect on cholesterol biosynthesis interferes with CAR activation. SIGNIFICANCE STATEMENT: The cholesterol biosynthesis inhibitor squalestatin 1 induces rat hepatic CYP2B expression indirectly by causing accumulation of an endogenous isoprenoid that activates the constitutive androstane receptor (CAR). This study demonstrates that squalestatin 1 does not similarly induce CYP2B6 expression in human hepatocytes. Rather, inhibition of cholesterol biosynthesis interferes with CAR activity, likely by activating sterol regulatory element binding proteins. These findings increase our understanding of the endogenous processes that modulate human drug-metabolizing gene expression.
Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
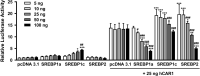

References
-
- Chen Y, Ferguson SS, Negishi M, Goldstein JA (2003) Identification of constitutive androstane receptor and glucocorticoid receptor binding sites in the CYP2C19 promoter. Mol Pharmacol 64:316–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

