Chromatin remodeler Arid1a regulates subplate neuron identity and wiring of cortical connectivity
- PMID: 34011608
- PMCID: PMC8166177
- DOI: 10.1073/pnas.2100686118
Chromatin remodeler Arid1a regulates subplate neuron identity and wiring of cortical connectivity
Abstract
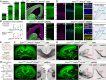
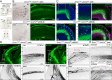
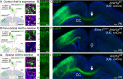
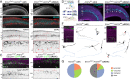
Loss-of-function mutations in chromatin remodeler gene ARID1A are a cause of Coffin-Siris syndrome, a developmental disorder characterized by dysgenesis of corpus callosum. Here, we characterize Arid1a function during cortical development and find unexpectedly selective roles for Arid1a in subplate neurons (SPNs). SPNs, strategically positioned at the interface of cortical gray and white matter, orchestrate multiple developmental processes indispensable for neural circuit wiring. We find that pancortical deletion of Arid1a leads to extensive mistargeting of intracortical axons and agenesis of corpus callosum. Sparse Arid1a deletion, however, does not autonomously misroute callosal axons, implicating noncell-autonomous Arid1a functions in axon guidance. Supporting this possibility, the ascending axons of thalamocortical neurons, which are not autonomously affected by cortical Arid1a deletion, are also disrupted in their pathfinding into cortex and innervation of whisker barrels. Coincident with these miswiring phenotypes, which are reminiscent of subplate ablation, we unbiasedly find a selective loss of SPN gene expression following Arid1a deletion. In addition, multiple characteristics of SPNs crucial to their wiring functions, including subplate organization, subplate axon-thalamocortical axon cofasciculation ("handshake"), and extracellular matrix, are severely disrupted. To empirically test Arid1a sufficiency in subplate, we generate a cortical plate deletion of Arid1a that spares SPNs. In this model, subplate Arid1a expression is sufficient for subplate organization, subplate axon-thalamocortical axon cofasciculation, and subplate extracellular matrix. Consistent with these wiring functions, subplate Arid1a sufficiently enables normal callosum formation, thalamocortical axon targeting, and whisker barrel development. Thus, Arid1a is a multifunctional regulator of subplate-dependent guidance mechanisms essential to cortical circuit wiring.
Keywords: axon pathfinding; cerebral cortex; chromatin regulation; development; neural circuits.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
Systemic and ocular manifestations of a patient with mosaic ARID1A-associated Coffin-Siris syndrome and review of select mosaic conditions with ophthalmic manifestations.Am J Med Genet C Semin Med Genet. 2020 Sep;184(3):644-655. doi: 10.1002/ajmg.c.31839. Epub 2020 Sep 5. Am J Med Genet C Semin Med Genet. 2020. PMID: 32888375 Free PMC article. Review.
-
Coffin-Siris syndrome is a SWI/SNF complex disorder.Clin Genet. 2014 Jun;85(6):548-54. doi: 10.1111/cge.12225. Epub 2013 Jul 23. Clin Genet. 2014. PMID: 23815551
-
A comprehensive molecular study on Coffin-Siris and Nicolaides-Baraitser syndromes identifies a broad molecular and clinical spectrum converging on altered chromatin remodeling.Hum Mol Genet. 2013 Dec 20;22(25):5121-35. doi: 10.1093/hmg/ddt366. Epub 2013 Aug 1. Hum Mol Genet. 2013. PMID: 23906836
-
Striking phenotypic overlap between Nicolaides-Baraitser and Coffin-Siris syndromes in monozygotic twins with ARID1B intragenic deletion.Eur J Med Genet. 2020 Mar;63(3):103739. doi: 10.1016/j.ejmg.2019.103739. Epub 2019 Aug 14. Eur J Med Genet. 2020. PMID: 31421289
-
Expanding the clinical spectrum of Coffin-Siris syndrome with anorectal malformations: Case report and review of the literature.Eur J Med Genet. 2024 Jun;69:104948. doi: 10.1016/j.ejmg.2024.104948. Epub 2024 May 10. Eur J Med Genet. 2024. PMID: 38735569 Review.
Cited by
-
PfARID Regulates P. falciparum Malaria Parasite Male Gametogenesis and Female Fertility and Is Critical for Parasite Transmission to the Mosquito Vector.mBio. 2022 Jun 28;13(3):e0057822. doi: 10.1128/mbio.00578-22. Epub 2022 May 31. mBio. 2022. PMID: 35638735 Free PMC article.
-
Tissue- and cell-type-specific molecular and functional signatures of 16p11.2 reciprocal genomic disorder across mouse brain and human neuronal models.Am J Hum Genet. 2022 Oct 6;109(10):1789-1813. doi: 10.1016/j.ajhg.2022.08.012. Epub 2022 Sep 23. Am J Hum Genet. 2022. PMID: 36152629 Free PMC article.
-
Postmitotic accumulation of histone variant H3.3 in new cortical neurons establishes neuronal chromatin, transcriptome, and identity.Proc Natl Acad Sci U S A. 2022 Aug 9;119(32):e2116956119. doi: 10.1073/pnas.2116956119. Epub 2022 Aug 5. Proc Natl Acad Sci U S A. 2022. PMID: 35930666 Free PMC article.
-
A facile method to generate cerebral organoids from human pluripotent stem cells.EXCLI J. 2023 Oct 5;22:1055-1076. doi: 10.17179/excli2023-6299. eCollection 2023. EXCLI J. 2023. PMID: 37927348 Free PMC article.
-
ARID1A serves as a receivable biomarker for the resistance to EGFR-TKIs in non-small cell lung cancer.Mol Med. 2021 Oct 29;27(1):138. doi: 10.1186/s10020-021-00400-5. Mol Med. 2021. PMID: 34715776 Free PMC article. Review.
References
-
- Allendoerfer K. L., Shatz C. J., The subplate, a transient neocortical structure: Its role in the development of connections between thalamus and cortex. Annu. Rev. Neurosci. 17, 185–218 (1994). - PubMed
-
- McConnell S. K., Ghosh A., Shatz C. J., Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science 245, 978–982 (1989). - PubMed
-
- Kostović I., Rakic P., Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J. Comp. Neurol. 297, 441–470 (1990). - PubMed
-
- Hoerder-Suabedissen A., Molnár Z., Development, evolution and pathology of neocortical subplate neurons. Nat. Rev. Neurosci. 16, 133–146 (2015). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

