CD28 engagement inhibits CD73-mediated regulatory activity of CD8+ T cells
- PMID: 34011962
- PMCID: PMC8134507
- DOI: 10.1038/s42003-021-02119-9
CD28 engagement inhibits CD73-mediated regulatory activity of CD8+ T cells
Abstract
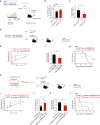
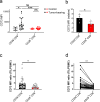
CD28 is required for T cell activation as well as the generation of CD4+Foxp3+ Treg. It is unclear, however, how CD28 costimulation affects the development of CD8+ T cell suppressive function. Here, by use of Hepa1.6.gp33 in vitro killing assay and B16.gp33 tumor mouse model we demonstrate that CD28 engagement during TCR ligation prevents CD8+ T cells from becoming suppressive. Interestingly, our results showed that ectonucleotidase CD73 expression on CD8+ T cells is upregulated in the absence of CD28 costimulation. In both murine and human tumor-bearing hosts, CD73 is upregulated on CD28-CD8+ T cells that infiltrate the solid tumor. UPLC-MS/MS analysis revealed that CD8+ T cells activation without CD28 costimulation produces elevated levels of adenosine and that CD73 mediates its production. Adenosine receptor antagonists block CD73-mediated suppression. Our data support the notion that CD28 costimulation inhibits CD73 upregulation and thereby prevents CD8+ T cells from becoming suppressive. This study uncovers a previously unidentified role for CD28 costimulation in CD8+ T cell activation and suggests that the CD28 costimulatory pathway can be a potential target for cancer immunotherapy.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Babcock SK, Gill RG, Bellgrau D, Lafferty KJ. Studies on the two-signal model for T cell activation in vivo. Transpl. Proc. 1987;19:303–306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

