Immunological Biomarkers of Fatal COVID-19: A Study of 868 Patients
- PMID: 34012444
- PMCID: PMC8126711
- DOI: 10.3389/fimmu.2021.659018
Immunological Biomarkers of Fatal COVID-19: A Study of 868 Patients
Abstract
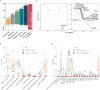
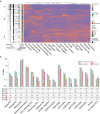
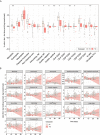
Information on the immunopathobiology of coronavirus disease 2019 (COVID-19) is rapidly increasing; however, there remains a need to identify immune features predictive of fatal outcome. This large-scale study characterized immune responses to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection using multidimensional flow cytometry, with the aim of identifying high-risk immune biomarkers. Holistic and unbiased analyses of 17 immune cell-types were conducted on 1,075 peripheral blood samples obtained from 868 COVID-19 patients and on samples from 24 patients presenting with non-SARS-CoV-2 infections and 36 healthy donors. Immune profiles of COVID-19 patients were significantly different from those of age-matched healthy donors but generally similar to those of patients with non-SARS-CoV-2 infections. Unsupervised clustering analysis revealed three immunotypes during SARS-CoV-2 infection; immunotype 1 (14% of patients) was characterized by significantly lower percentages of all immune cell-types except neutrophils and circulating plasma cells, and was significantly associated with severe disease. Reduced B-cell percentage was most strongly associated with risk of death. On multivariate analysis incorporating age and comorbidities, B-cell and non-classical monocyte percentages were independent prognostic factors for survival in training (n=513) and validation (n=355) cohorts. Therefore, reduced percentages of B-cells and non-classical monocytes are high-risk immune biomarkers for risk-stratification of COVID-19 patients.
Keywords: COVID-19; SARS-CoV-2; biomarkers; flow cytometry; lymphopenia; outcome; survival.
Copyright © 2021 Martín-Sánchez, Garcés, Maia, Inogés, López-Díaz de Cerio, Carmona-Torre, Marin-Oto, Alegre, Molano, Fernandez-Alonso, Perez, Botta, Zabaleta, Alcaide, Landecho, Rua, Pérez-Warnisher, Blanco, Sarvide, Vilas-Zornoza, Alignani, Moreno, Pineda, Sogbe, Argemi, Paiva and Yuste.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

