Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia: Who, When, and How?
- PMID: 34012445
- PMCID: PMC8126705
- DOI: 10.3389/fimmu.2021.659595
Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia: Who, When, and How?
Abstract
Although the majority of patients with acute myeloid leukemia (AML) treated with intensive chemotherapy achieve a complete remission (CR), many are destined to relapse if treated with intensive chemotherapy alone. Allogeneic stem cell transplant (allo-SCT) represents a pivotally important treatment strategy in fit adults with AML because of its augmented anti-leukemic activity consequent upon dose intensification and the genesis of a potent graft-versus-leukemia effect. Increased donor availability coupled with the advent of reduced intensity conditioning (RIC) regimens has dramatically increased transplant access and consequently allo-SCT is now a key component of the treatment algorithm in both patients with AML in first CR (CR1) and advanced disease. Although transplant related mortality has fallen steadily over recent decades there has been no real progress in reducing the risk of disease relapse which remains the major cause of transplant failure and represents a major area of unmet need. A number of therapeutic approaches with the potential to reduce disease relapse, including advances in induction chemotherapy, the development of novel conditioning regimens and the emergence of the concept of post-transplant maintenance, are currently under development. Furthermore, the use of genetics and measurable residual disease technology in disease assessment has improved the identification of patients who are likely to benefit from an allo-SCT which now represents an increasingly personalized therapy. Future progress in optimizing transplant outcome will be dependent on the successful delivery by the international transplant community of randomized prospective clinical trials which permit examination of current and future transplant therapies with the same degree of rigor as is routinely adopted for non-transplant therapies.
Keywords: acute myeloid leukemia; allogeneic stem cell transplantation; chemotherapy; graft-versus-leukemia; measurable residual disease (MRD); minimal residual disease.
Copyright © 2021 Loke, Buka and Craddock.
Conflict of interest statement
CC has received honoraria from Celgene, Daichi-Sankyo, Novartis and Pfizer as well as research funding from Celgene. JL has received travel funding from Novartis and Daichi-Sankyo, honoraria from Pfizer, Janssen and Amgen. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer CS has declared past co-authorships with one of the authors CC, to the handling editor, at the time of review.
Figures
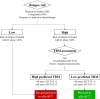


References
-
- Cornelissen JJ, Breems D, Putten WLJV, Gratwohl AA, Passweg JR, Pabst T, et al. . Comparative Analysis of the Value of Allogeneic Hematopoietic Stem-Cell Transplantation in Acute Myeloid Leukemia With Monosomal Karyotype Versus Other Cytogenetic Risk Categories. J Clin Oncol (2012) 30(17):2140–6. 10.1200/JCO.2011.39.6499 - DOI - PubMed
-
- Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, et al. . A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council’s Adult and Childhood Leukaemia Working Parties. Br J Haematol (1999) 107(1):69–79. 10.1046/j.1365-2141.1999.01684.x - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

