Differentiation and Regulation of TH Cells: A Balancing Act for Cancer Immunotherapy
- PMID: 34012451
- PMCID: PMC8126720
- DOI: 10.3389/fimmu.2021.669474
Differentiation and Regulation of TH Cells: A Balancing Act for Cancer Immunotherapy
Abstract
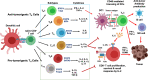
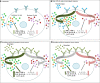
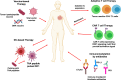
Current success of immunotherapy in cancer has drawn attention to the subsets of TH cells in the tumor which are critical for activation of anti-tumor response either directly by themselves or by stimulating cytotoxic T cell activity. However, presence of immunosuppressive pro-tumorigenic TH subsets in the tumor milieu further contributes to the complexity of regulation of TH cell-mediated immune response. In this review, we present an overview of the multifaceted positive and negative effects of TH cells, with an emphasis on regulation of different TH cell subtypes by various immune cells, and how a delicate balance of contradictory signals can influence overall success of cancer immunotherapy. We focus on the regulatory network that encompasses dendritic cell-induced activation of CD4+ TH1 cells and subsequent priming of CD8+ cytotoxic T cells, along with intersecting anti-inflammatory and pro-tumorigenic TH2 cell activity. We further discuss how other tumor infiltrating immune cells such as immunostimulatory TH9 and Tfh cells, immunosuppressive Treg cells, and the duality of TH17 function contribute to tip the balance of anti- vs pro-tumorigenic TH responses in the tumor. We highlight the developing knowledge of CD4+ TH1 immune response against neoantigens/oncodrivers, impact of current immunotherapy strategies on CD4+ TH1 immunity, and how opposing action of TH cell subtypes can be explored further to amplify immunotherapy success in patients. Understanding the nuances of CD4+ TH cells regulation and the molecular framework undergirding the balancing act between anti- vs pro-tumorigenic TH subtypes is critical for rational designing of immunotherapies that can bypass therapeutic escape to maximize the potential of immunotherapy.
Keywords: CD4; T helper; immunotherapy; neoantigen; tumor associated antigen.
Copyright © 2021 Basu, Ramamoorthi, Albert, Gallen, Beyer, Snyder, Koski, Disis, Czerniecki and Kodumudi.
Conflict of interest statement
BC and GK have patent application filed for intellectual property on a human version of DC1. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

