Stage III N2 non-small cell lung cancer treatment: decision-making among surgeons and radiation oncologists
- PMID: 34012806
- PMCID: PMC8107728
- DOI: 10.21037/tlcr-20-1210
Stage III N2 non-small cell lung cancer treatment: decision-making among surgeons and radiation oncologists
Abstract
Background: Stage III N2 non-small cell lung cancer (NSCLC) is a very heterogeneous disease associated with a poor prognosis. A number of therapeutic options are available for patients with Stage III N2 NSCLC, including surgery [with neoadjuvant or adjuvant chemotherapy (CTx)/neoadjuvant chemoradiotherapy (CRT)] or CRT potentially followed by adjuvant immunotherapy. We have no clear evidence demonstrating a significant survival benefit for either of these approaches, the selection between treatments is not always straightforward and can come down to physician and patient preference. The very heterogeneous definition of resectability of N2 disease makes the decision-making process even more complex.
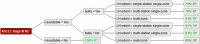
Methods: We evaluated the treatment strategies for preoperatively diagnosed stage III cN2 NSCLC among Swiss thoracic surgeons and radiation oncologists. Treatment strategies were converted into decision trees and analysed for consensus and discrepancies. We analysed factors relevant to decision-making within these recommendations.
Results: For resectable "non-bulky" mediastinal lymph node involvement, there was a trend towards surgery. Numerous participants recommend a surgical approach outside existing guidelines as long as the disease was resectable, even in multilevel N2. With increasing extent of mediastinal nodal disease, multimodal treatment based on radiotherapy was more common.
Conclusions: Both, surgery- or radiotherapy-based treatment regimens are feasible options in the management of Stage III N2 NSCLC. The different opinions reflected in the results of this manuscript reinforce the importance of a multidisciplinary setting and the importance of shared decision-making with the patient.
Keywords: Non-small cell lung cancer (NSCLC); decision-making; radiotherapy; stage III N2 lung cancer; surgery.
2021 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-1210). The series “Radiotherapy in thoracic malignancies” was commissioned by the editorial office without any funding or sponsorship. PMP received an educational grant from AstraZeneca (educational grant to the Institution); outside the submitted work, he also received research support and educational grants to the department from Celgene, Roche and Takeda. The authors have no other conflicts of interest to declare.
Figures



References
-
- Eberhardt WE, Pottgen C, Gauler TC, et al. Phase III Study of Surgery Versus Definitive Concurrent Chemoradiotherapy Boost in Patients With Resectable Stage IIIA(N2) and Selected IIIB Non-Small-Cell Lung Cancer After Induction Chemotherapy and Concurrent Chemoradiotherapy (ESPATUE). J Clin Oncol 2015;33:4194-201. 10.1200/JCO.2015.62.6812 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
