Efficacy and Safety of Pyrotinib Versus T-DM1 in HER2+ Metastatic Breast Cancer Patients Pre-Treated With Trastuzumab and a Taxane: A Bayesian Network Meta-Analysis
- PMID: 34012912
- PMCID: PMC8127838
- DOI: 10.3389/fonc.2021.608781
Efficacy and Safety of Pyrotinib Versus T-DM1 in HER2+ Metastatic Breast Cancer Patients Pre-Treated With Trastuzumab and a Taxane: A Bayesian Network Meta-Analysis
Abstract
Purpose: To compare the efficacy and safety between pyrotinib (Pyr) and trastuzumab emtansine (T-DM1) in pre-treated human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (MBC) patients.
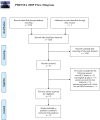
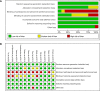
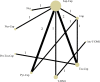
Methods: A comprehensive literature search of the PubMed, EMBASE, and Web of Science was performed in August 2020. Randomized clinical trials comparing the efficacy and safety between different anti-HER2 regimens in patients pre-treated with trastuzumab (Tra) and a taxane in metastatic settings (≤second-line treatment) were included. A fixed effects network meta-analysis based on the Bayesian inferential framework was conducted for progression-free survival (PFS), overall survival (OS), overall response rate (ORR), and grade ≥3 adverse events (AEs). Values of surface under cumulative ranking probability curve (SUCRA) were calculated to offer a ranking of all regimens.
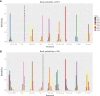
Results: Twelve studies with 4,353 subjects were identified. Nine regimens were included into the network: T-DM1, lapatinib-capecitabine (Lap-Cap), Tra-Cap, Cap, neratinib (Ner), pertuzumab (Per)-Tra-Cap, Pyr-Cap, atezolizumab (Ate)-T-DM1, and Ner-Cap. For PFS, Pyr-Cap was more favorable than T-DM1 (hazard ratio, 95% confidence interval: 0.77, 0.70-0.86), Lap-Cap (0.64, 0.59-0.69), Tra-Cap (0.63, 0.56-0.70), Cap (0.50, 0.45-0.56), Ner (0.59, 0.51-0.69), Per-Tra-Cap (0.68, 0.59-0.79), and Ner-Cap (0.72, 0.64-0.81). For OS, Pyr-Cap showed further improvement than Lap-Cap (hazard ratio, 95% confidence interval: 0.71, 0.52-0.99), Cap (0.68, 0.49-0.96), and Ner (0.65, 0.45-0.94). For ORR, Pyr-Cap was significantly superior than Cap (odds ratio, 95% confidence interval: 7.87, 1.22-56.51). No significant difference was observed in grade ≥3 AEs among all the regimens. Pyr-Cap ranked in the highest in PFS, OS, ORR, and grade ≥3 AEs (SUCRA = 99.4, 89.7, 86.4, and 89.3%).
Conclusions: These results indicate that Pyr may be more effective than T-DM1 in HER2+ MBC patients pre-treated with Tra and a taxane. However, it may be associated with more grade ≥3 AEs.
Keywords: human epidermal growth factor; metastatic breast cancer; network meta-analysis; pyrotinib; trastuzumab emtansine.
Copyright © 2021 Liao, Huang, Liu, Pei and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Primary results of ELAINA: a randomized, multicenter, open-label, phase III study of the efficacy and safety of trastuzumab emtansine vs. lapatinib plus capecitabine in Chinese patients with HER2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab-based therapy.Transl Breast Cancer Res. 2023 Jan 31;4:3. doi: 10.21037/tbcr-23-2. eCollection 2023. Transl Breast Cancer Res. 2023. PMID: 38751488 Free PMC article.
-
HER2-targeted regimens after prior trastuzumab for patients with HER2-positive unresectable, locally advanced or metastatic breast cancer: a network meta-analysis of randomized controlled trials.Ann Transl Med. 2020 Dec;8(24):1634. doi: 10.21037/atm-20-5149. Ann Transl Med. 2020. PMID: 33490146 Free PMC article.
-
Evaluating the clinical effectiveness and safety of various HER2-targeted regimens after prior taxane/trastuzumab in patients with previously treated, unresectable, or metastatic HER2-positive breast cancer: a systematic review and network meta-analysis.Breast Cancer Res Treat. 2020 Apr;180(3):597-609. doi: 10.1007/s10549-020-05577-7. Epub 2020 Feb 25. Breast Cancer Res Treat. 2020. PMID: 32100144 Free PMC article.
-
Efficacy of tucatinib for HER2-positive metastatic breast cancer after HER2-targeted therapy: a network meta-analysis.Future Oncol. 2021 Nov;17(33):4635-4647. doi: 10.2217/fon-2021-0742. Epub 2021 Aug 31. Future Oncol. 2021. PMID: 34463120
-
Metastatic Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Current Treatment Standards and Future Perspectives.Breast Care (Basel). 2020 Dec;15(6):570-578. doi: 10.1159/000512328. Epub 2020 Nov 12. Breast Care (Basel). 2020. PMID: 33447230 Free PMC article. Review.
Cited by
-
Relative efficacy of antibody-drug conjugates and other anti-HER2 treatments on survival in HER2-positive advanced breast cancer: a systematic review and meta-analysis.BMC Cancer. 2024 Jun 8;24(1):708. doi: 10.1186/s12885-024-12478-1. BMC Cancer. 2024. PMID: 38851684 Free PMC article.
-
Clinical updates on tyrosine kinase inhibitors in HER2-positive breast cancer.Front Pharmacol. 2022 Dec 12;13:1089066. doi: 10.3389/fphar.2022.1089066. eCollection 2022. Front Pharmacol. 2022. PMID: 36578543 Free PMC article. Review.
-
Efficacy and safety of neoadjuvant pyrotinib plus docetaxel/liposomal doxorubicin/cyclophosphamide for HER2-positive breast cancer.Ir J Med Sci. 2023 Jun;192(3):1041-1049. doi: 10.1007/s11845-022-03093-9. Epub 2022 Jul 13. Ir J Med Sci. 2023. PMID: 35829909
-
Endocrine therapy plus HER2-targeted therapy, another favorable option for HR+/HER2+ advanced breast cancer patients.Ther Adv Med Oncol. 2024 Jan 6;16:17588359231220501. doi: 10.1177/17588359231220501. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 38188468 Free PMC article. Review.
-
Primary results of ELAINA: a randomized, multicenter, open-label, phase III study of the efficacy and safety of trastuzumab emtansine vs. lapatinib plus capecitabine in Chinese patients with HER2-positive locally advanced or metastatic breast cancer who have received prior trastuzumab-based therapy.Transl Breast Cancer Res. 2023 Jan 31;4:3. doi: 10.21037/tbcr-23-2. eCollection 2023. Transl Breast Cancer Res. 2023. PMID: 38751488 Free PMC article.
References
-
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for Subtypes–Dealing With the Diversity of Breast Cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol (2011) 22(8):1736–47. 10.1093/annonc/mdr304 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

