Efficacy and Safety of Pyrotinib Versus T-DM1 in HER2+ Metastatic Breast Cancer Patients Pre-Treated With Trastuzumab and a Taxane: A Bayesian Network Meta-Analysis
- PMID: 34012912
- PMCID: PMC8127838
- DOI: 10.3389/fonc.2021.608781
Efficacy and Safety of Pyrotinib Versus T-DM1 in HER2+ Metastatic Breast Cancer Patients Pre-Treated With Trastuzumab and a Taxane: A Bayesian Network Meta-Analysis
Abstract
Purpose: To compare the efficacy and safety between pyrotinib (Pyr) and trastuzumab emtansine (T-DM1) in pre-treated human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (MBC) patients.
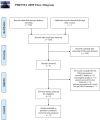
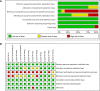
Methods: A comprehensive literature search of the PubMed, EMBASE, and Web of Science was performed in August 2020. Randomized clinical trials comparing the efficacy and safety between different anti-HER2 regimens in patients pre-treated with trastuzumab (Tra) and a taxane in metastatic settings (≤second-line treatment) were included. A fixed effects network meta-analysis based on the Bayesian inferential framework was conducted for progression-free survival (PFS), overall survival (OS), overall response rate (ORR), and grade ≥3 adverse events (AEs). Values of surface under cumulative ranking probability curve (SUCRA) were calculated to offer a ranking of all regimens.
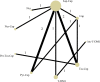
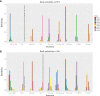
Results: Twelve studies with 4,353 subjects were identified. Nine regimens were included into the network: T-DM1, lapatinib-capecitabine (Lap-Cap), Tra-Cap, Cap, neratinib (Ner), pertuzumab (Per)-Tra-Cap, Pyr-Cap, atezolizumab (Ate)-T-DM1, and Ner-Cap. For PFS, Pyr-Cap was more favorable than T-DM1 (hazard ratio, 95% confidence interval: 0.77, 0.70-0.86), Lap-Cap (0.64, 0.59-0.69), Tra-Cap (0.63, 0.56-0.70), Cap (0.50, 0.45-0.56), Ner (0.59, 0.51-0.69), Per-Tra-Cap (0.68, 0.59-0.79), and Ner-Cap (0.72, 0.64-0.81). For OS, Pyr-Cap showed further improvement than Lap-Cap (hazard ratio, 95% confidence interval: 0.71, 0.52-0.99), Cap (0.68, 0.49-0.96), and Ner (0.65, 0.45-0.94). For ORR, Pyr-Cap was significantly superior than Cap (odds ratio, 95% confidence interval: 7.87, 1.22-56.51). No significant difference was observed in grade ≥3 AEs among all the regimens. Pyr-Cap ranked in the highest in PFS, OS, ORR, and grade ≥3 AEs (SUCRA = 99.4, 89.7, 86.4, and 89.3%).
Conclusions: These results indicate that Pyr may be more effective than T-DM1 in HER2+ MBC patients pre-treated with Tra and a taxane. However, it may be associated with more grade ≥3 AEs.
Keywords: human epidermal growth factor; metastatic breast cancer; network meta-analysis; pyrotinib; trastuzumab emtansine.
Copyright © 2021 Liao, Huang, Liu, Pei and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for Subtypes–Dealing With the Diversity of Breast Cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol (2011) 22(8):1736–47. 10.1093/annonc/mdr304 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

