Hydrogel Scaffolds to Deliver Cell Therapies for Wound Healing
- PMID: 34012956
- PMCID: PMC8126987
- DOI: 10.3389/fbioe.2021.660145
Hydrogel Scaffolds to Deliver Cell Therapies for Wound Healing
Abstract
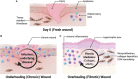
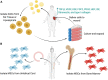
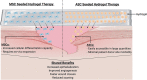
Cutaneous wounds are a growing global health burden as a result of an aging population coupled with increasing incidence of diabetes, obesity, and cancer. Cell-based approaches have been used to treat wounds due to their secretory, immunomodulatory, and regenerative effects, and recent studies have highlighted that delivery of stem cells may provide the most benefits. Delivering these cells to wounds with direct injection has been associated with low viability, transient retention, and overall poor efficacy. The use of bioactive scaffolds provides a promising method to improve cell therapy delivery. Specifically, hydrogels provide a physiologic microenvironment for transplanted cells, including mechanical support and protection from native immune cells, and cell-hydrogel interactions may be tailored based on specific tissue properties. In this review, we describe the current and future directions of various cell therapies and usage of hydrogels to deliver these cells for wound healing applications.
Keywords: cell therapy; fibrosis; hydrogel; stem cell; wound healing.
Copyright © 2021 Sivaraj, Chen, Chattopadhyay, Henn, Wu, Noishiki, Magbual, Mittal, Mermin-Bunnell, Bonham, Trotsyuk, Barrera, Padmanabhan, Januszyk and Gurtner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

