Systematic Review and Meta-analysis of Herpes Zoster Vaccine in Patients With CKD
- PMID: 34013103
- PMCID: PMC8116755
- DOI: 10.1016/j.ekir.2021.02.024
Systematic Review and Meta-analysis of Herpes Zoster Vaccine in Patients With CKD
Abstract
Introduction: Chronic kidney disease (CKD) is a risk factor for herpes zoster (HZ) infection. Few studies have examined HZ vaccine (HZV) in this population. We conducted a systematic review and meta-analysis investigating the efficacy and safety of HZV in patients with renal disease (CKD, dialysis, and transplant).
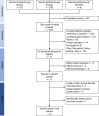
Methods: MEDLINE, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases (up to May 2020) were searched for randomized controlled trials and nonrandomized controlled studies evaluating HZV in patients with CKD for effectiveness and adverse event risks. Studies without a control group (placebo or no vaccine) were excluded. Extraction of prespecified data and risk of bias assessments using the Newcastle-Ottawa scale for cohort studies and the Cochrane Risk of Bias Tool for randomized controlled trials were done by 3 authors. Random-effects meta-analysis was used to generate pooled treatment effects and 95% confidence intervals.
Results: Included were 404,561 individuals from 8 studies (3 randomized controlled trials and 5 nonrandomized). All 8 studies examined HZ as an outcome, with 3 reporting adverse events. Risk of HZ was lower in patients who received HZV compared with controls (hazard ratio, 0.55; 95% confidence interval, 0.37-0.82; P < 0.01); however, heterogeneity was high (I 2 = 88%, P < 0.01). There was no significant difference in adverse events associated with HZV (hazard ratio, 1.03; 95% confidence interval, 0.54-1.28; P = 0.8).
Conclusions: HZV compared with control significantly lowers the risk of HZ without an increase in adverse events in CKD patients. However, significant heterogeneity was present. HZV should be actively considered in CKD patients because the prevalence of HZ is higher in this population.
Keywords: chronic kidney disease; dialysis; herpes zoster; herpes zoster vaccine; meta-analysis; transplant.
© 2021 International Society of Nephrology. Published by Elsevier Inc.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources

