Molecular, functional, and pathological aspects of TDP-43 fragmentation
- PMID: 34013172
- PMCID: PMC8113996
- DOI: 10.1016/j.isci.2021.102459
Molecular, functional, and pathological aspects of TDP-43 fragmentation
Abstract
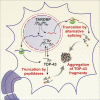
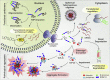
Transactive response DNA binding protein 43 (TDP-43) is a DNA/RNA binding protein involved in transcriptional regulation and RNA processing. It is linked to sporadic and familial amyotrophic lateral sclerosis and frontotemporal lobar degeneration. TDP-43 is predominantly nuclear, but it translocates to the cytoplasm under pathological conditions. Cytoplasmic accumulation, phosphorylation, ubiquitination and truncation of TDP-43 are the main hallmarks of TDP-43 proteinopathies. Among these processes, the pathways leading to TDP-43 fragmentation remain poorly understood. We review here the molecular and biochemical properties of several TDP-43 fragments, the mechanisms and factors mediating their production, and their potential role in disease progression. We also address the presence of TDP-43 C-terminal fragments in several neurological disorders, including Alzheimer's disease, and highlight their respective implications. Finally, we discuss features of animal models expressing TDP-43 fragments as well as recent therapeutic strategies to approach TDP-43 truncation.
Keywords: Biological sciences; Molecular biology; Molecular interaction; Molecular physiology.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Pathological hallmarks of amyotrophic lateral sclerosis/frontotemporal lobar degeneration in transgenic mice produced with TDP-43 genomic fragments.Brain. 2011 Sep;134(Pt 9):2610-26. doi: 10.1093/brain/awr159. Epub 2011 Jul 13. Brain. 2011. PMID: 21752789
-
TDP-43 proteinopathies: neurodegenerative protein misfolding diseases without amyloidosis.Neurosignals. 2008;16(1):41-51. doi: 10.1159/000109758. Epub 2007 Dec 5. Neurosignals. 2008. PMID: 18097159 Review.
-
TDP-43 cytoplasmic inclusion formation is disrupted in C9orf72-associated amyotrophic lateral sclerosis/frontotemporal lobar degeneration.Brain Commun. 2019;1(1):fcz014. doi: 10.1093/braincomms/fcz014. Epub 2019 Sep 11. Brain Commun. 2019. PMID: 31633109 Free PMC article.
-
[A new dementia group caused by TDP-43 abnormality].Seishin Shinkeigaku Zasshi. 2011;113(6):574-83. Seishin Shinkeigaku Zasshi. 2011. PMID: 21815469 Review. Japanese.
-
Expression of TDP-43 C-terminal Fragments in Vitro Recapitulates Pathological Features of TDP-43 Proteinopathies.J Biol Chem. 2009 Mar 27;284(13):8516-24. doi: 10.1074/jbc.M809462200. Epub 2009 Jan 21. J Biol Chem. 2009. PMID: 19164285 Free PMC article.
Cited by
-
Adolescent mice exposed to TBI developed PD-like pathology in middle age.Transl Psychiatry. 2025 Jan 25;15(1):27. doi: 10.1038/s41398-025-03232-7. Transl Psychiatry. 2025. PMID: 39863574 Free PMC article.
-
Single Acetylation-mimetic Mutation in TDP-43 Nuclear Localization Signal Disrupts Importin α1/β Signaling.J Mol Biol. 2024 Oct 15;436(20):168751. doi: 10.1016/j.jmb.2024.168751. Epub 2024 Aug 22. J Mol Biol. 2024. PMID: 39181183
-
Emerging Roles of lncRNAs Regulating RNA-Mediated Type-I Interferon Signaling Pathway.Front Immunol. 2022 Feb 25;13:811122. doi: 10.3389/fimmu.2022.811122. eCollection 2022. Front Immunol. 2022. PMID: 35280983 Free PMC article. Review.
-
Different Chronic Stress Paradigms Converge on Endogenous TDP43 Cleavage and Aggregation.Mol Neurobiol. 2023 Nov;60(11):6346-6361. doi: 10.1007/s12035-023-03455-z. Epub 2023 Jul 14. Mol Neurobiol. 2023. PMID: 37450246 Free PMC article.
-
Comprehensive analysis of co-expressed genes with TDP-43: prognostic and therapeutic potential in lung adenocarcinoma.J Cancer Res Clin Oncol. 2024 Jan 28;150(2):44. doi: 10.1007/s00432-023-05554-9. J Cancer Res Clin Oncol. 2024. PMID: 38281298 Free PMC article.
References
-
- Afroz T., Hock E.-M., Ernst P., Foglieni C., Jambeau M., Gilhespy L.A.B., Laferriere F., Maniecka Z., Plückthun A., Mittl P. Functional and dynamic polymerization of the ALS-linked protein TDP-43 antagonizes its pathologic aggregation. Nat. Commun. 2017;8:45. doi: 10.1038/s41467-017-00062-0. - DOI - PMC - PubMed
-
- Alves S., Marais T., Biferi M.-G., Furling D., Marinello M., Hachimi K.E., Cartier N., Ruberg M., Stevanin G., Brice A. Lentiviral vector-mediated overexpression of mutant ataxin-7 recapitulates SCA7 pathology and promotes accumulation of the FUS/TLS and MBNL1 RNA-binding proteins. Mol. Neurodegener. 2016;11:58. doi: 10.1186/s13024-016-0123-2. - DOI - PMC - PubMed
-
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. - DOI - PubMed
-
- Archbold H.C., Jackson K.L., Arora A., Weskamp K., Tank E.M.-H., Li X., Miguez R., Dayton R.D., Tamir S., Klein R.L. TDP43 nuclear export and neurodegeneration in models of amyotrophic lateral sclerosis and frontotemporal dementia. Sci. Rep. 2018;8:4606. doi: 10.1038/s41598-018-22858-w. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

