Repetitive Blast Exposure Produces White Matter Axon Damage without Subsequent Myelin Remodeling: In Vivo Analysis of Brain Injury Using Fluorescent Reporter Mice
- PMID: 34013219
- PMCID: PMC8127063
- DOI: 10.1089/neur.2020.0058
Repetitive Blast Exposure Produces White Matter Axon Damage without Subsequent Myelin Remodeling: In Vivo Analysis of Brain Injury Using Fluorescent Reporter Mice
Abstract
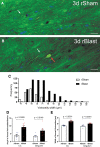
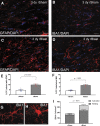
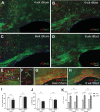
The potential effects of blast exposure on the brain health of military personnel have raised concerns and led to increased surveillance of blast exposures. Neuroimaging studies have reported white matter abnormalities in brains of service members with a history of blast exposure. However, blast effects on white matter microstructure remain poorly understood. As a novel approach to screen for white matter effects, transgenic mice that express fluorescent reporters to sensitively detect axon damage and myelin remodeling were exposed to simulated repetitive blasts (once/day on 5 consecutive days). Axons were visualized using Thy1-YFP-16 reporter mice that express yellow fluorescent protein (YFP) in a broad spectrum of neurons. Swelling along damaged axons forms varicosities that fill with YFP. The frequency and size of axonal varicosities were significantly increased in the corpus callosum (CC) and cingulum at 3 days after the final blast exposure, versus in sham procedures. CC immunolabeling for reactive astrocyte and microglial markers was also significantly increased. NG2CreER;mTmG mice were given tamoxifen (TMX) on days 2 and 3 after the final blast to induce fluorescent labeling of newly synthesized myelin membranes, indicating plasticity and/or repair. Myelin synthesis was not altered in the CC over the intervening 4 or 8 weeks after repetitive blast exposure. These experiments show the advantages of transgenic reporter mice for analysis of white matter injury that detects subtle, diffuse axon damage and the dynamic nature of myelin sheaths. These results show that repetitive low-level blast exposures produce infrequent but significant axon damage along with neuroinflammation in white matter.
Keywords: axon damage; blast exposure; myelin; transgenic reporter mice; traumatic brain injury; white matter.
© Donald V. Bradshaw Jr et al., 2021; Published by Mary Ann Liebert, Inc.
Conflict of interest statement
No competing financial interests exist.
Figures

References
-
- Mac Donald, C.L., Barber, J., Patterson, J., Johnson, A.M., Dikmen, S., Fann, J.R., and Temkin, N. (2019). Association between 5-year clinical outcome in patients with nonmedically evacuated mild blast traumatic brain injury and clinical measures collected within 7 days postinjury in combat. JAMA Netw. Open 2, e186676. - PMC - PubMed
-
- Aldag, M., Armstrong, R.C., Bandak, F., Bellgowan, P.S.F., Bentley, T., Biggerstaff, S., Caravelli, K., Cmarik, J., Crowder, A., DeGraba, T.J., Dittmer, T.A., Ellenbogen, R.G., Greene, C., Gupta, R.K., Hicks, R., Hoffman, S., Latta, R.C., 3rd, Leggieri, M.J.Jr., Marion, D., Mazzoli, R., McCrea, M., O'Donnell, J., Packer, M., Petro, J.B., Rasmussen, T.E., Sammons-Jackson, W., Shoge, R., Tepe, V., Tremaine, L.A., and Zheng, J. (2017). The biological basis of chronic traumatic encephalopathy following blast injury: a literature review. J. Neurotrauma 34, S26–S43 - PMC - PubMed
-
- Regasa, L.E., Agimi, Y., and Stout, K.C. (2019). Traumatic brain injury following military deployment: evaluation of diagnosis and cause of injury. J. Head Trauma Rehabil. 34, 21–29 - PubMed
-
- Engel, C.C., Hoch, E., Simmons, M.M. (2019). The neurological effects of repeated exposure to military occupational levels of blast: a review of scientific literature. RAND Conference Proceedings
-
- Carr, W., Stone, J.R., Walilko, T., Young, L.A., Snook, T.L., Paggi, M.E., Tsao, J.W., Jankosky, C.J., Parish, R.V., and Ahlers, S.T. (2016). Repeated low-level blast exposure: a descriptive human subjects study. Mil Med. 181, 28–39 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
