This is a preprint.
Modeling SARS-CoV-2 and Influenza Infections and Antiviral Treatments in Human Lung Epithelial Tissue Equivalents
- PMID: 34013274
- PMCID: PMC8132232
- DOI: 10.1101/2021.05.11.443693
Modeling SARS-CoV-2 and Influenza Infections and Antiviral Treatments in Human Lung Epithelial Tissue Equivalents
Update in
-
Modeling SARS-CoV-2 and influenza infections and antiviral treatments in human lung epithelial tissue equivalents.Commun Biol. 2022 Aug 12;5(1):810. doi: 10.1038/s42003-022-03753-7. Commun Biol. 2022. PMID: 35962146 Free PMC article.
Abstract
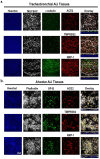
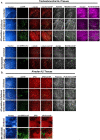
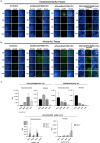
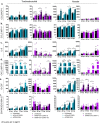
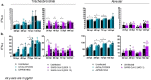
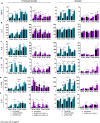
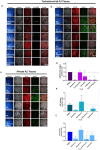
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the third coronavirus in less than 20 years to spillover from an animal reservoir and cause severe disease in humans. High impact respiratory viruses such as pathogenic beta-coronaviruses and influenza viruses, as well as other emerging respiratory viruses, pose an ongoing global health threat to humans. There is a critical need for physiologically relevant, robust and ready to use, in vitro cellular assay platforms to rapidly model the infectivity of emerging respiratory viruses and discover and develop new antiviral treatments. Here, we validate in vitro human alveolar and tracheobronchial tissue equivalents and assess their usefulness as in vitro assay platforms in the context of live SARS-CoV-2 and influenza A virus infections. We establish the cellular complexity of two distinct tracheobronchial and alveolar epithelial air liquid interface (ALI) tissue models, describe SARS-CoV-2 and influenza virus infectivity rates and patterns in these ALI tissues, the viral-induced cytokine production as it relates to tissue-specific disease, and demonstrate the pharmacologically validity of these lung epithelium models as antiviral drug screening assay platforms.
Conflict of interest statement
Conflict of Interest
There are no conflicts of interest to report.
Figures
Similar articles
-
Modeling SARS-CoV-2 and influenza infections and antiviral treatments in human lung epithelial tissue equivalents.Commun Biol. 2022 Aug 12;5(1):810. doi: 10.1038/s42003-022-03753-7. Commun Biol. 2022. PMID: 35962146 Free PMC article.
-
Toll-Like Receptor 3 Signaling via TRIF Contributes to a Protective Innate Immune Response to Severe Acute Respiratory Syndrome Coronavirus Infection.mBio. 2015 May 26;6(3):e00638-15. doi: 10.1128/mBio.00638-15. mBio. 2015. PMID: 26015500 Free PMC article.
-
Human Organotypic Airway and Lung Organoid Cells of Bronchiolar and Alveolar Differentiation Are Permissive to Infection by Influenza and SARS-CoV-2 Respiratory Virus.Front Cell Infect Microbiol. 2022 Mar 14;12:841447. doi: 10.3389/fcimb.2022.841447. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35360113 Free PMC article.
-
Eight influenza virus cellular manipulations which can boost concurrent SARS-CoV-2 infections to severe outcomes.Hum Cell. 2023 Sep;36(5):1581-1592. doi: 10.1007/s13577-023-00923-5. Epub 2023 Jun 12. Hum Cell. 2023. PMID: 37306884 Review.
-
Broad-spectrum coronavirus antiviral drug discovery.Expert Opin Drug Discov. 2019 Apr;14(4):397-412. doi: 10.1080/17460441.2019.1581171. Epub 2019 Mar 8. Expert Opin Drug Discov. 2019. PMID: 30849247 Free PMC article. Review.
Cited by
-
Molecular mechanisms of transgenerational epigenetic inheritance.Nat Rev Genet. 2022 Jun;23(6):325-341. doi: 10.1038/s41576-021-00438-5. Epub 2022 Jan 4. Nat Rev Genet. 2022. PMID: 34983971 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
