A causal role for the pulvinar in coordinating task-independent cortico-cortical interactions
- PMID: 34013540
- PMCID: PMC8556184
- DOI: 10.1002/cne.25193
A causal role for the pulvinar in coordinating task-independent cortico-cortical interactions
Erratum in
-
Erratum.J Comp Neurol. 2022 May;530(7):1126. doi: 10.1002/cne.25315. J Comp Neurol. 2022. PMID: 35338485 Free PMC article. No abstract available.
Abstract
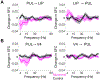
The pulvinar is the largest nucleus in the primate thalamus and has topographically organized connections with multiple cortical areas, thereby forming extensive cortico-pulvino-cortical input-output loops. Neurophysiological studies have suggested a role for these transthalamic pathways in regulating information transmission between cortical areas. However, evidence for a causal role of the pulvinar in regulating cortico-cortical interactions is sparse and it is not known whether pulvinar's influences on cortical networks are task-dependent or, alternatively, reflect more basic large-scale network properties that maintain functional connectivity across networks regardless of active task demands. In the current study, under passive viewing conditions, we conducted simultaneous electrophysiological recordings from ventral (area V4) and dorsal (lateral intraparietal area [LIP]) nodes of macaque visual system, while reversibly inactivating the dorsal part of the lateral pulvinar (dPL), which shares common anatomical connectivity with V4 and LIP, to probe a causal role of the pulvinar. Our results show a significant reduction in local field potential phase coherence between LIP and V4 in low frequencies (4-15 Hz) following muscimol injection into dPL. At the local level, no significant changes in firing rates or LFP power were observed in LIP or in V4 following dPL inactivation. Synchronization between pulvinar spikes and cortical LFP phase decreased in low frequencies (4-15 Hz) both in LIP and V4, while the low frequency synchronization between LIP spikes and pulvinar phase increased. These results indicate a causal role for pulvinar in synchronizing neural activity between interconnected cortical nodes of a large-scale network, even in the absence of an active task state.
Keywords: cortico-cortical interactions; macaque electrophysiology; pulvinar causal role; reversible-inactivation with muscimol; transthalamic pathways.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
Conflict of interest statement
The authors declare no conflicts of interests
Figures



References
-
- Adams MM, Hof PR, Gattass R, Webster MJ, & Ungerleider LG (2000). Visual cortical projections and chemoarchitecture of macaque monkey pulvinar. Journal of Comparative Neurology, 419, 377–393. - PubMed
-
- Andersson JLR, Skare S, & Ashburner J (2003). How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage, 20, 870–888. - PubMed
-
- Arcelli P, Frassoni C, Regondi MC, Biasi SD, & Spreafico R (1997). GABAergic neurons in mammalian thalamus: A marker of thalamic complexity? Brain Research Bulletin, 42, 27–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

