Molecular mechanism of Mad1 kinetochore targeting by phosphorylated Bub1
- PMID: 34013668
- PMCID: PMC8391104
- DOI: 10.15252/embr.202052242
Molecular mechanism of Mad1 kinetochore targeting by phosphorylated Bub1
Abstract
During metaphase, in response to improper kinetochore-microtubule attachments, the spindle assembly checkpoint (SAC) activates the mitotic checkpoint complex (MCC), an inhibitor of the anaphase-promoting complex/cyclosome (APC/C). This process is orchestrated by the kinase Mps1, which initiates the assembly of the MCC onto kinetochores through a sequential phosphorylation-dependent signalling cascade. The Mad1-Mad2 complex, which is required to catalyse MCC formation, is targeted to kinetochores through a direct interaction with the phosphorylated conserved domain 1 (CD1) of Bub1. Here, we present the crystal structure of the C-terminal domain of Mad1 (Mad1CTD ) bound to two phosphorylated Bub1CD1 peptides at 1.75 Å resolution. This interaction is mediated by phosphorylated Bub1 Thr461, which not only directly interacts with Arg617 of the Mad1 RLK (Arg-Leu-Lys) motif, but also directly acts as an N-terminal cap to the CD1 α-helix dipole. Surprisingly, only one Bub1CD1 peptide binds to the Mad1 homodimer in solution. We suggest that this stoichiometry is due to inherent asymmetry in the coiled-coil of Mad1CTD and has implications for how the Mad1-Bub1 complex at kinetochores promotes efficient MCC assembly.
Keywords: Bub1; Cell cycle; Mad1; Mitotic checkpoint complex; Spindle assembly checkpoint.
© 2021 MRC Laboratory of Molecular Biology. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Outline of the essential sequential steps of MCC assembly onto the outer kinetochore.
A model of MCC assembly onto the outer kinetochore. MCC assembly occurs in a stepwise manner that is under the control of Mps1 and Cdk1 kinases. Several MELT motifs on Knl1 are first phosphorylated by Mps1. pMELTs then recruit the Bub3:Bub1 complex which is then phosphorylated first by Cdk1 then by Mps1 on the Bub1 CD1 domain. Phosphorylated Bub1 then recruits the Mad1:C‐Mad2 complex which then acts as a platform for O‐Mad2 binding and catalyst for conversion into C‐Mad2. Bub1 is likely responsible for Cdc20 targeting to kinetochores through its KEN and ABBA motifs as well BubR1 through a central Bub1‐BubR1 dimerization domain. The CTD of Mad1 is phosphorylated by Mps1 which interacts with an N‐terminal tail of Cdc20. Bub1 therefore acts as a scaffold to position C‐Mad2, BubR1 and Cdc20 in close proximity for efficient MCC formation. Once formed soluble MCC then binds and inactivates the APC/C, preventing metaphase to anaphase progression.

Schematics of full‐length human Mad1 and Bub1. The domains crystallized in this study are highlighted by dashed boxes. A sequence conservation map produced by ClustalX2 is shown for the Bub1CD1 domain. The two sites of phosphorylation (pSer459/pThr461) are highlighted by black arrows. A1: ABBA motif. K1/2: KEN box motifs. MIM: Mad2 interacting motif. CD1: conserved domain 1. BDD: Bub dimerization domain. GLEBS: Gle2‐binding‐sequence. TPR: Tetratricopeptide repeat. RLK: Arg‐Leu‐Lys motif. RWD: RING, WD40, DEAD domain.
Three views of the crystal structure of Mad1CTD homodimer (dark/light orange) bound to two Bub1CD1 peptides (purple/blue). The RLK motif of Mad1 is highlighted in yellow. The two phosphorylation sites are shown as sticks.

The extensive largely hydrophobic interface of the Mad1CTD‐Bub1CD1 interaction is highlighted with the higher occupancy CD1 peptide (purple). The RLK motif of Mad1 is emphasized in yellow.
Close‐up view of Bub1CD1 interactions with the head domain of Mad1CTD. Hydrogen bonding interactions within 3.5 Å are highlighted by black dashes.
Close‐up view of the Mad1 Arg617 and Bub1 pThr461 interaction. An additional contact occurs between the phosphate of pThr461 and Bub1 His463 which then forms a hydrogen bond with Mad1 Ser610. Additional stabilizing hydrogen bonding occurs between the pThr461 phosphate and the amide nitrogen atoms of Val462, His463 and Thr464. Hydrogen bonding interactions within 3.5 Å are highlighted by black dashes.
Top view of the conserved RLK motifs of the Mad1 homodimer which are shown as sticks. The sidechains of hydrophobic residues near the RLK site at the surface of Bub1CD1 are shown as purple sticks which form a hydrophobic pocket.

Isothermal calorimetry (ITC) of Mad1CTD binding to doubly phosphorylated Bub1CD1. Bub1CD1 was injected into Mad1CTD in 19 injections of 2 µl, revealing a dissociation constant (kD) of approximately 2.7 µM and a stoichiometry of 1:1 Mad1CTD dimer to Bub1CD1 peptide.
Summary of all ITC experiments performed in this study. The KD and stoichiometry (n) values were obtained by averaging at least three experiments. The reported error values are calculated standard deviations. The mutations of Mad1CTD are highlighted in the Bub1CD1‐Mad1CTD crystal structure in (C). Mutants which do not bind are marked with a dash. Raw data for each ITC reaction are shown in Appendix Figs S5, S6 and S8. SpT* CD1 peptide is a peptide which is phosphorylated at Thr461 and not at Ser459. QRI** and QRRI** are triple and quadruple mutants of Mad1 which contact the C‐terminus of Bub1CD1. The QRI** triple mutant contains Q627A, R650A and I643A, while the QRRI** quadruple mutant additionally contains R630A.
The crystal structure of Bub1CD1‐Mad1CTD with the Mad1 residues which were mutated in our ITC experiments shown as sticks. Residues from both monomers are shown. The lower occupancy peptide is shown as blue and the higher occupancy peptide as purple.
31P 1D spectra showing phosphorylated Bub1CD1 peptide titrated with an increasing concentration of Mad1CTD dimer. The peptide sequence is shown above with the two phosphorylated residues highlighted in red. Peaks corresponding to pSer459 and pThr461 are marked with red arrows. The major pSer459 peak represents pSer459 in a trans Ser‐Pro bond, and the minor pSer459 peak (marked grey) represents pSer459 in a cis Ser‐Pro bond. In the 1:1 molar ratio of Mad1CTD dimer to Bub1CD1, the pThr461 peak is significantly line broadened and the pSer459 peak (marked blue) is perturbed. In the 1:2 molar ratio of Mad1CTD dimer to Bub1CD1, in addition to signal for the bound pSer459, there is reappearance of the original free position of pS459 and unbound pThr461 supporting the presence of unbound peptide.

Size‐exclusion multi‐angle light scattering (SEC‐MALS) of Mad1CTD wild‐type and L618A and F629A mutants. All eluted as monodispersed species. The average mass of WT Mad1CTD was 26.2 kDa. The average mass for the L618A and F629A mutants was 29.6 and 26.7 kDa, respectively. A tailing peak of about 23 kDa most likely comes from residual amount of TEV protease in the sample.
Analytical ultracentrifugation sedimentation equilibrium of Mad1CTD‐Bub1CD1 complexes. Samples were run in triplicate. The data were fitted to a two‐species model where the mass of the Bub1CD1 peptide with an N‐terminal tryptophan residue (3,247 Da) was fixed. Standard errors are shown.

A snapshot of the electron density map of Mad1CTD‐Bub1CD1 visualized in Coot (Emsley et al, 2010) which shows the differential Bub1CD1 peptide occupancy. Both Mad1CTD subunits are depicted as ribbons in shades of blue. Bub1CD1_high has higher occupancy and is represented as purple throughout this paper. Bub1CD1_low has lower occupancy and is represented as blue.
Electron density map visualized in Coot for a lower resolution Mad1CTD‐Bub1CD1 structure showing more equivalent peptide occupancy (Emsley et al, 2010). Two times the peptide concentration as compared to the complex crystallized in (A) was used during co‐crystallization (5 mM total) requiring 10% DMSO.
Electron density map for each peptide is shown using Isomesh in PYMOL. The same threshold for each peptide is displayed. Differences between the sidechain density and positioning of each peptide can be seen, as well as clear phosphate density for both pThr461 phosphates.
The 2Fo‐Fc omit map (green) for both Bub1CD1 peptides in the Bub1CD1‐Mad1CTD crystal structure. Created with Phenix (Liebschner et al, 2019).
The extensive interface of the Mad1CTD‐Bub1CD1 interaction is highlighted with the lower occupancy Bub1CD1 peptide (blue). The RLK motif of Mad1CTD is coloured yellow.
Close‐up view of the lower occupancy Bub1CD1 peptide (blue) interactions with the head domain of Mad1CTD (orange/light orange). Hydrogen bonding interactions within 3.5 Å are highlighted by black dashes.
Close‐up view of the Mad1CTD Arg617 and Bub1CD1 pThr461 interaction in the lower occupancy peptide (blue). Hydrogen bonding interactions within 3.5 Å are highlighted by black dashes. Additional contact occurs between the phosphate of pThr461 and Bub1CD1 His463 which then forms a hydrogen bond with Mad1CTD Ser610. Additional stabilizing hydrogen bonding occurs between the pThr461 phosphate and the amide nitrogen of Val462, His463 and Thr464.

Crystal structure of apo Mad1CTD (grey) with the RLK motif highlighted in yellow. PDB ID: 4DZO (Kim et al, 2012).
Bottom view of the crystal structure of apo Mad1CTD showing the extent of the bending of apoA helix.
Opposite subunits of two copies of apo Mad1CTD homodimer aligned onto their respective RLK motifs (yellow). One copy is in grey, the other in black. The arrows highlight the rotation of the head domain inwards towards the inside of the coiled‐coil curvature.
Alignment of opposite subunits of the head domain of two apo Mad1CTD copies. One copy is in grey, the other in black.
Apo Mad1CTD coloured by relative B‐factors. The more bent helix (apoA) and its adjacent head domain (apoB_head) exhibit higher flexibility.

Alignment of the RLK motif of opposite subunits of the Mad1CTD‐Bub1CD1 structure. The site of alignment is highlighted by the dashed box. In one dimer (coloured orange), the higher occupancy peptide is depicted as an orange cartoon. In the other dimer (coloured blue), the lower occupancy peptide is depicted as a blue cartoon. The orange and blue arrows highlight how there is stronger engagement of the head domain with the higher occupancy peptide.
Alignment of the head domain of opposite subunits of the Mad1CTD‐Bub1CD1 structure. In one dimer (coloured orange), the higher occupancy peptide is depicted as an orange cartoon. In the other dimer (coloured blue), the lower occupancy peptide is depicted as a blue cartoon.
Comparison of the high and low occupancy Bub1CD1 peptide contacts with the opposite sides of the Mad1 head domain and top of the coiled‐coil. Hydrogen bonds within 3.5 Å are shown as black dashes.
Temperature factors mapped onto the Mad1CTD‐Bub1CD1 crystal structure.
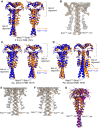
Alignment of Mad1CTD‐Bub1CD1 structure from the P21212 space group (PDB: 7B1J). Left panel: Alignment of opposite subunits of the homodimer on the RLK site. Right panel: Alignment of opposite subunits on the head domain. The duplicated dimers are coloured in orange or blue.
Electron density map of the Mad1CTD‐Bub1CD1 structure from the P21212 space group (PDB: 7B1J) visualized in Coot (Emsley et al, 2010) which shows near equivalent peptide occupancy. The Mad1CTD homodimer is represented as a ribbon in orange and yellow.
Alignment of one homodimer from the Mad1CTD‐Bub1CD1 structure from the P21 space group (PDB: 7B1H). Left panel: Alignment of opposite subunits of the homodimer on the RLK site. Right panel: Alignment of opposite subunits on the head domain. The duplicated dimers are coloured in orange or blue.
Alignment of the second homodimer from the Mad1CTD‐Bub1CD1 structure from the P21 space group (PDB: 7B1H). Left panel: Alignment of opposite subunits of the homodimer on the RLK site. Right panel: Alignment of opposite subunits on the head domain. The duplicated dimers are coloured in orange or blue.
Electron density map of one homodimer from the Mad1CTD‐Bub1CD1 structure (shown directly above) from P21 space group visualized in Coot (Emsley et al, 2010). The Mad1CTD homodimer is represented as a ribbon in green and yellow.
Electron density map of the second homodimer from the Mad1CTD‐Bub1CD1 structure (shown directly above) from the P21 space group visualized in Coot (Emsley et al, 2010). The Mad1CTD homodimer is represented as a ribbon in pink and blue. The occupancy of the peptide on the right is extremely poor as is the density for the head domain it contacts.
All four Mad1CTD homodimers from the three different space group structures are aligned onto their Mad1CTD RLK motif which is bound to the higher occupancy peptide. Blue = homodimer_1 from P21. Orange = homodimer_2 from P21. Purple = P212121 homodimer. Black = P21212.

1H,15N‐2D HSQC showing 15N‐labelled Mad1CTD with (blue) and without (grey) Bub1 phosphorylated peptide. Peptides were added in excess at 1:2 molar ratio of Mad1CTD dimer to Bub1CD1. Assignments of the backbone resonances of Mad1CTD are labelled on the spectra. Close‐up of the chemical shift perturbations of the residues in red during Bub1CD1 titration, are shown in Appendix Figs S7 and S8.
Relative peak intensities (bound/free) of Mad1CTD upon Bub1CD1 binding. Peak intensities were normalized to that of the C‐terminal residue Ala718.
Relative peak intensities of Bub1CD1‐bound Mad1CTD in B are mapped onto the Bub1CD1‐Mad1CTD crystal structure. Residues are coloured in a scale of blue to grey, where regions with the most significant line broadening are highlighted in blue.
15N{1H}‐heteronuclear NOE values were collected with interleaved on‐ (I) and off‐ (I0) resonance and expressed as I/I0. A higher value indicates higher rigidity of the backbone N–H bond. The Mad1 RLK motif is highlighted in red. The error bars are the calculated standard deviations of two technical replicates on the same sample.
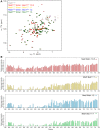
1H,15N‐2D HSQC showing 15N‐labelled Mad1CTD with an increasing concentration of phosphorylated Bub1CD1 peptide. In molar ratios of Mad1CTD dimer to Bub1CD1 peptides were added at 1:0.5 (red), 1:1 (yellow), 1:2 (blue) and 1:4 (green) ratios.
Relative peak intensities of Mad1CTD upon titration of phosphorylated Bub1CD1 peptides. The bar charts follow a similar colour scheme as the spectra in A, with molar ratios of Mad1CTD dimer to Bub1CD1 at 1:0.5 (red), 1:1 (yellow), 1:2 (blue) and 1:4 (green) ratios. Peak intensities were normalized to that of the C‐terminal residue Ala718.
Similar articles
-
Juxtaposition of Bub1 and Cdc20 on phosphorylated Mad1 during catalytic mitotic checkpoint complex assembly.Nat Commun. 2022 Oct 26;13(1):6381. doi: 10.1038/s41467-022-34058-2. Nat Commun. 2022. PMID: 36289199 Free PMC article.
-
Conserved signalling functions for Mps1, Mad1 and Mad2 in the Cryptococcus neoformans spindle checkpoint.PLoS Genet. 2024 Jun 3;20(6):e1011302. doi: 10.1371/journal.pgen.1011302. eCollection 2024 Jun. PLoS Genet. 2024. PMID: 38829899 Free PMC article.
-
Direct interactions of mitotic arrest deficient 1 (MAD1) domains with each other and MAD2 conformers are required for mitotic checkpoint signaling.J Biol Chem. 2018 Jan 12;293(2):484-496. doi: 10.1074/jbc.RA117.000555. Epub 2017 Nov 21. J Biol Chem. 2018. PMID: 29162720 Free PMC article.
-
Orchestration of the spindle assembly checkpoint by CDK1-cyclin B1.FEBS Lett. 2019 Oct;593(20):2889-2907. doi: 10.1002/1873-3468.13591. Epub 2019 Sep 13. FEBS Lett. 2019. PMID: 31469407 Review.
-
Kinetochore structure and function.Trends Cell Biol. 2005 Nov;15(11):589-98. doi: 10.1016/j.tcb.2005.09.010. Epub 2005 Oct 7. Trends Cell Biol. 2005. PMID: 16214339 Review.
Cited by
-
Spindle assembly checkpoint activation and silencing at kinetochores.Semin Cell Dev Biol. 2021 Sep;117:86-98. doi: 10.1016/j.semcdb.2021.06.009. Epub 2021 Jun 29. Semin Cell Dev Biol. 2021. PMID: 34210579 Free PMC article. Review.
-
Role of spindle assembly checkpoint proteins in gametogenesis and embryogenesis.Front Cell Dev Biol. 2025 Jan 22;12:1491394. doi: 10.3389/fcell.2024.1491394. eCollection 2024. Front Cell Dev Biol. 2025. PMID: 39911185 Free PMC article. Review.
-
Aurora B phosphorylates Bub1 to promote spindle assembly checkpoint signaling.Curr Biol. 2022 Jan 10;32(1):237-247.e6. doi: 10.1016/j.cub.2021.10.049. Epub 2021 Dec 2. Curr Biol. 2022. PMID: 34861183 Free PMC article.
-
Interplay of kinetochores and catalysts drives rapid assembly of the mitotic checkpoint complex.Nat Commun. 2025 May 24;16(1):4823. doi: 10.1038/s41467-025-59970-1. Nat Commun. 2025. PMID: 40410156 Free PMC article.
-
Functional dissection of human mitotic genes using CRISPR-Cas9 tiling screens.Genes Dev. 2022 Apr 1;36(7-8):495-510. doi: 10.1101/gad.349319.121. Epub 2022 Apr 28. Genes Dev. 2022. PMID: 35483740 Free PMC article.
References
-
- Brady DM, Hardwick KG (2000) Complex formation between Mad1p, Bub1p and Bub3p is crucial for spindle checkpoint function. Curr Biol 10: 675–678 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

