Extracellular acidosis enhances Zika virus infection both in human cells and ex-vivo tissue cultures from female reproductive tract
- PMID: 34013833
- PMCID: PMC8205022
- DOI: 10.1080/22221751.2021.1932606
Extracellular acidosis enhances Zika virus infection both in human cells and ex-vivo tissue cultures from female reproductive tract
Abstract
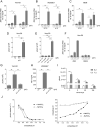
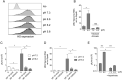
Zika virus (ZIKV) is a flavivirus transmitted by mosquitoes of the genus Aedes, but unlike other flaviviruses, ZIKV can be sexually transmitted by vaginal intercourse. The healthy vaginal pH ranges from 4.0 to 6.0, reaching values of 6.0-7.0 after semen deposition. Here, we report that low extracellular pH values (range 6.2-6.6) dramatically increase ZIKV infection on cell lines of different origin including some derived from the female genital tract and monocyte-derived macrophages. Furthermore, low pH significantly increased ZIKV infection of human ectocervix and endocervix cultured ex-vivo. Enhancement of infection by low pH was also observed using different ZIKV strains and distinct methods to evaluate viral infection, i.e. plaque assays, RT-PCR, flow cytometry, and fluorescence microscopy. Analysis of the mechanisms involved revealed that the enhancement of ZIKV infection induced by low pH was associated with increased binding of the viral particles to the heparan sulphate expressed on the target cell surface. Acidosis represents a critical but generally overlooked feature of the female genital tract, with major implications for sexual transmission diseases. Our results suggest that low vaginal pH might promote male-to-female transmission of ZIKV infection.
Keywords: ZIKV; ex-vivo tissues; extracellular acidosis; heparan sulphate; sexual transmission.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Brasil P, Calvet GA, de Souza RV, et al. . Exanthema associated with Zika virus infection. Lancet Infect Dis. 2016;16:866. - PubMed
-
- Honein MA, Dawson AL, Petersen EE, et al. . Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317:59–68. - PubMed
-
- Mlakar J, Korva M, Tul N, et al. . Zika virus associated with microcephaly. N Engl J Med. 2016;374:951–958. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
