Multiple Switches From the Originator Infliximab to Biosimilars Is Effective and Safe in Inflammatory Bowel Disease: A Prospective Multicenter Cohort Study
- PMID: 34013959
- PMCID: PMC8972297
- DOI: 10.1093/ibd/izab099
Multiple Switches From the Originator Infliximab to Biosimilars Is Effective and Safe in Inflammatory Bowel Disease: A Prospective Multicenter Cohort Study
Abstract
Background: Though a single nonmedical switch from the originator infliximab (IFX) to a biosimilar is considered effective and safe for most patients with inflammatory bowel disease (IBD), very limited data are available on multiple successive switches.
Methods: We performed a prospective multicenter cohort study of adult IBD patients who underwent 2 switches from the originator IFX to CT-P13 to SB2 (group 1), 1 switch from CT-P13 to SB2 (group 2), and 1 switch from the originator IFX to CT-P13 (group 3). Patients were assessed at 4 and 12 months since the most recent switch for remission using clinical (physician's assessment) and biochemical (C-reactive protein [CRP], and fecal calprotectin [FC]) measures. Patients discontinuing treatment for ineffectiveness or adverse events before month 12 were imputed as nonremitters.
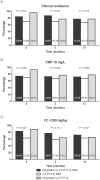
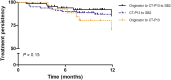
Results: One hundred seventy-six patients (Crohn's disease 71%, ulcerative colitis 27.8%, IBD unclassified 1.2%; group 1, 69; group 2, 80; group 3, 27) were included. At 12 months after the most recent switch 76.9% (40 of 52, group 1), 65.7% (46 of 70, group 2) and 76.9% (20 of 26, group 3) of patients were in clinical remission. Treatment persistence at 12 months was 85.0%, 87.0%, and 70.1%, respectively. There were no significant differences in the rate of clinical, CRP, FC remission, or treatment persistence at 12 months between the 3 groups. Infusion reactions occurred in 1.7% of patients (3/176), all in patients with antidrug antibodies from group 2.
Conclusions: Multiple successive switching and switching between biosimilars of IFX seemed to be effective and safe.
Keywords: CT-P13; SB2; multiple switches.
© 2021 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Figures
References
-
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613–1620. - PMC - PubMed
-
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605–1612. - PMC - PubMed
-
- Choe JY, Prodanovic N, Niebrzydowski J, et al. A randomised, double-blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product Remicade in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017;76:58–64. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

