Pertactin-Deficient Bordetella pertussis, Vaccine-Driven Evolution, and Reemergence of Pertussis
- PMID: 34014152
- PMCID: PMC8153889
- DOI: 10.3201/eid2706.203850
Pertactin-Deficient Bordetella pertussis, Vaccine-Driven Evolution, and Reemergence of Pertussis
Abstract
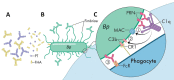
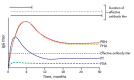
Recent reemergence of pertussis (whooping cough) in highly vaccinated populations and rapid expansion of Bordetella pertussis strains lacking pertactin (PRN), a common acellular vaccine antigen, have raised the specter of vaccine-driven evolution and potential return of what was once the major killer of children. The discovery that most circulating B. pertussis strains in the United States have acquired new and independent disruptive mutations in PRN is compelling evidence of strong selective pressure. However, the other 4 antigens included in acellular vaccines do not appear to be selected against so rapidly. We consider 3 aspects of PRN that distinguish it from other vaccine antigens, which might, individually or collectively, explain why only this antigen is being precipitously eliminated. An understanding of the increase in PRN-deficient strains should provide useful information for the current search for new protective antigens and provide broader lessons for the design of improved subunit vaccines.
Keywords: Bordetella pertussis; PRN; United States; acellular vaccine; antibody titers; bacteria; pertactin; pertactin deficient; pertussis; reemergence; respiratory infections; vaccine-driven evolution; vaccines; waning immunity; whole-cell vaccine; whooping cough.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

