Influenza Vaccine Effectiveness for Prevention of Severe Influenza-Associated Illness Among Adults in the United States, 2019-2020: A Test-Negative Study
- PMID: 34014274
- PMCID: PMC8682606
- DOI: 10.1093/cid/ciab462
Influenza Vaccine Effectiveness for Prevention of Severe Influenza-Associated Illness Among Adults in the United States, 2019-2020: A Test-Negative Study
Abstract
Background: Influenza vaccine effectiveness (VE) against a spectrum of severe disease, including critical illness and death, remains poorly characterized.
Methods: We conducted a test-negative study in an intensive care unit (ICU) network at 10 US hospitals to evaluate VE for preventing influenza-associated severe acute respiratory infection (SARI) during the 2019-2020 season, which was characterized by circulation of drifted A/H1N1 and B-lineage viruses. Cases were adults hospitalized in the ICU and a targeted number outside the ICU (to capture a spectrum of severity) with laboratory-confirmed, influenza-associated SARI. Test-negative controls were frequency-matched based on hospital, timing of admission, and care location (ICU vs non-ICU). Estimates were adjusted for age, comorbidities, and other confounders.
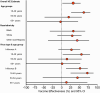
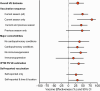
Results: Among 638 patients, the median (interquartile) age was 57 (44-68) years; 286 (44.8%) patients were treated in the ICU and 42 (6.6%) died during hospitalization. Forty-five percent of cases and 61% of controls were vaccinated, which resulted in an overall VE of 32% (95% CI: 2-53%), including 28% (-9% to 52%) against influenza A and 52% (13-74%) against influenza B. VE was higher in adults 18-49 years old (62%; 95% CI: 27-81%) than those aged 50-64 years (20%; -48% to 57%) and ≥65 years old (-3%; 95% CI: -97% to 46%) (P = .0789 for interaction). VE was significantly higher against influenza-associated death (80%; 95% CI: 4-96%) than nonfatal influenza illness.
Conclusions: During a season with drifted viruses, vaccination reduced severe influenza-associated illness among adults by 32%. VE was high among young adults.
Keywords: critical illness; immunization; influenza; vaccination; vaccine effectiveness.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Seasonal influenza vaccine effectiveness against laboratory-confirmed influenza hospitalizations - Latin America, 2013.Vaccine. 2018 Jun 7;36(24):3555-3566. doi: 10.1016/j.vaccine.2017.06.036. Epub 2017 Jun 23. Vaccine. 2018. PMID: 28648543 Free PMC article.
-
Influenza vaccine effectiveness against influenza-related hospitalization during a season with mixed outbreaks of four influenza viruses: a test-negative case-control study in adults in Canada.BMC Infect Dis. 2017 Dec 29;17(1):805. doi: 10.1186/s12879-017-2905-8. BMC Infect Dis. 2017. PMID: 29284435 Free PMC article.
-
Vaccine Effectiveness Against Acute Respiratory Illness Hospitalizations for Influenza-Associated Pneumonia During the 2015-2016 to 2017-2018 Seasons: US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN).Clin Infect Dis. 2022 Apr 28;74(8):1329-1337. doi: 10.1093/cid/ciab654. Clin Infect Dis. 2022. PMID: 34320171 Free PMC article.
-
Early estimates of seasonal influenza vaccine effectiveness - United States, January 2015.MMWR Morb Mortal Wkly Rep. 2015 Jan 16;64(1):10-5. MMWR Morb Mortal Wkly Rep. 2015. PMID: 25590680 Free PMC article.
-
Influenza vaccine effectiveness against influenza-associated hospitalization in children: A systematic review and meta-analysis.Vaccine. 2020 Mar 23;38(14):2893-2903. doi: 10.1016/j.vaccine.2020.02.049. Epub 2020 Feb 27. Vaccine. 2020. PMID: 32113808
Cited by
-
Rapid whole-blood assay to detect SARS-CoV-2-specific memory T-cell immunity following a single dose of AstraZeneca ChAdOx1-S COVID-19 vaccine.Clin Transl Immunology. 2021 Aug 12;10(8):e1326. doi: 10.1002/cti2.1326. eCollection 2021. Clin Transl Immunology. 2021. PMID: 34408875 Free PMC article.
-
Vaccine Effectiveness Against Influenza-Associated Urgent Care, Emergency Department, and Hospital Encounters During the 2021-2022 Season, VISION Network.J Infect Dis. 2023 Jul 14;228(2):185-195. doi: 10.1093/infdis/jiad015. J Infect Dis. 2023. PMID: 36683410 Free PMC article.
-
Disentangling the relative importance of T cell responses in COVID-19: leading actors or supporting cast?Nat Rev Immunol. 2022 Jun;22(6):387-397. doi: 10.1038/s41577-022-00716-1. Epub 2022 Apr 28. Nat Rev Immunol. 2022. PMID: 35484322 Free PMC article. Review.
-
Effectiveness of SARS-CoV-2 mRNA Vaccines for Preventing Covid-19 Hospitalizations in the United States.medRxiv [Preprint]. 2021 Jul 8:2021.07.08.21259776. doi: 10.1101/2021.07.08.21259776. medRxiv. 2021. Update in: Clin Infect Dis. 2022 May 3;74(9):1515-1524. doi: 10.1093/cid/ciab687. PMID: 34268515 Free PMC article. Updated. Preprint.
-
The Impact of Coronavirus Disease 2019 on Viral, Bacterial, and Fungal Respiratory Infections.Clin Chest Med. 2023 Jun;44(2):407-423. doi: 10.1016/j.ccm.2022.11.018. Epub 2023 Feb 26. Clin Chest Med. 2023. PMID: 37085229 Free PMC article. Review.
References
-
- Chung JR, Rolfes MA, Flannery B, et al. ; US Influenza Vaccine Effectiveness Network; Influenza Hospitalization Surveillance Network; Assessment Branch, Immunization Services Division, Centers for Disease Control and Prevention . Effects of influenza vaccination in the United States during the 2018–2019 influenza season. Clin Infect Dis 2020; 71:e368–76. - PubMed
-
- Rolfes MA, Flannery B, Chung JR, et al. ; US Influenza Vaccine Effectiveness (Flu VE) Network; Influenza Hospitalization Surveillance Network; Assessment Branch, Immunization Services Division, Centers for Disease Control and Prevention . Effects of influenza vaccination in the United States during the 2017–2018 influenza season. Clin Infect Dis 2019; 69:1845–53. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

