Ocean Acidification Amplifies the Olfactory Response to 2-Phenylethylamine: Altered Cue Reception as a Mechanistic Pathway?
- PMID: 34014453
- PMCID: PMC8613125
- DOI: 10.1007/s10886-021-01276-9
Ocean Acidification Amplifies the Olfactory Response to 2-Phenylethylamine: Altered Cue Reception as a Mechanistic Pathway?
Abstract
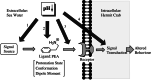
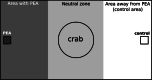
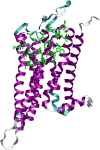
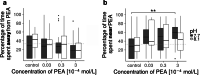
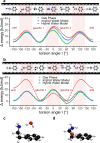
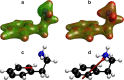
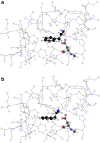
With carbon dioxide (CO2) levels rising dramatically, climate change threatens marine environments. Due to increasing CO2 concentrations in the ocean, pH levels are expected to drop by 0.4 units by the end of the century. There is an urgent need to understand the impact of ocean acidification on chemical-ecological processes. To date, the extent and mechanisms by which the decreasing ocean pH influences chemical communication are unclear. Combining behaviour assays with computational chemistry, we explore the function of the predator related cue 2-phenylethylamine (PEA) for hermit crabs (Pagurus bernhardus) in current and end-of-the-century oceanic pH. Living in intertidal environments, hermit crabs face large pH fluctuations in their current habitat in addition to climate-change related ocean acidification. We demonstrate that the dietary predator cue PEA for mammals and sea lampreys is an attractant for hermit crabs, with the potency of the cue increasing with decreasing pH levels. In order to explain this increased potency, we assess changes to PEA's conformational and charge-related properties as one potential mechanistic pathway. Using quantum chemical calculations validated by NMR spectroscopy, we characterise the different protonation states of PEA in water. We show how protonation of PEA could affect receptor-ligand binding, using a possible model receptor for PEA (human TAAR1). Investigating potential mechanisms of pH-dependent effects on olfactory perception of PEA and the respective behavioural response, our study advances the understanding of how ocean acidification interferes with the sense of smell and thereby might impact essential ecological interactions in marine ecosystems.
Keywords: Chemically-mediated behaviour; Chemoattractant; DFT; Ligand protonation; Pagurus bernhardus; TAAR1 receptor.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Adamo C, Barone V. Toward reliable density functional methods without adjustable parameters: The pbe0 model. J Chem Phys. 1999;110(13):6158–6170.
-
- Anderson K, Mathis A. Friends in low places: Responses of a bethic stream fish to intra-prey-guild alarm cues. Ethology. 2016;122(12):954–962.
-
- Andujar S, Tosso R, Suvire F, Angelina E, Peruchena N, Cabedo N, Cortes D, Enriz R. Searching the “biologically relevant” conformation of dopamine: a computational approach. J Chem Inf Model. 2011;52(1):99–112. - PubMed
-
- Barone V, Cossi M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A. 1998;102(11):1995–2001.
-
- Barroso N, Rodriguez M. Action of β-phenylethylamine and related amines on nigrostriatal dopamine neurotransmission. European J Pharmacol. 1996;297(3):195–203. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

