A Family of Superhelicenes: Easily Tunable, Chiral Nanographenes by Merging Helicity with Planar π Systems
- PMID: 34014601
- PMCID: PMC8456895
- DOI: 10.1002/anie.202103253
A Family of Superhelicenes: Easily Tunable, Chiral Nanographenes by Merging Helicity with Planar π Systems
Abstract
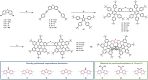
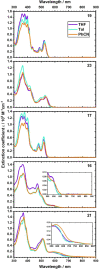
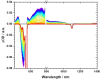
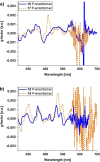
We designed a straightforward synthetic route towards a full-fledged family of π-extended helicenes: superhelicenes. They have two hexa-peri-hexabenzocoronenes (HBCs) in common that are connected via a central five-membered ring. By means of structurally altering this 5-membered ring, we realized a versatile library of molecular building blocks. Not only the superhelicene structure, but also their features are tuned with ease. In-depth physico-chemical characterizations served as a proof of concept thereof. The superhelicene enantiomers were separated, their circular dichroism was measured in preliminary studies and concluded with an enantiomeric assignment. Our work was rounded-off by crystal structure analyses. Mixed stacks of M- and P-isomers led to twisted molecular wires. Using such stacks, charge-carrier mobilities were calculated, giving reason to expect outstanding hole transporting properties.
Keywords: circular dichroism; helicenes; luminescence; nanographene; polycyclic aromatic hydrocarbons.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

