In Vitro and In Vivo Comparison of 3,2-HOPO Versus Deferoxamine-Based Chelation of Zirconium-89 to the Antimesothelin Antibody Anetumab
- PMID: 34014767
- PMCID: PMC8161658
- DOI: 10.1089/cbr.2020.4492
In Vitro and In Vivo Comparison of 3,2-HOPO Versus Deferoxamine-Based Chelation of Zirconium-89 to the Antimesothelin Antibody Anetumab
Abstract
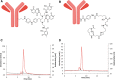
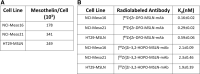
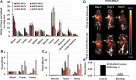
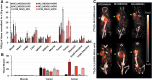
Introduction: [227Th]Th-3,2-HOPO-MSLN-mAb, a mesothelin (MSLN)-targeted thorium-227 therapeutic conjugate, is currently in phase I clinical trial; however, direct PET imaging using this conjugate is technically challenging. Thus, using the same MSLN antibody, we synthesized 3,2-HOPO and deferoxamine (DFO)-based zirconium-89 antibody conjugates, [89Zr]Zr-3,2-HOPO-MSLN-mAb and [89Zr]Zr-DFO-MSLN-mAb, respectively, and compared them in vitro and in vivo. Methods: [89Zr]Zr-3,2-HOPO-MSLN-mAb and [89Zr]Zr-DFO-MSLN-mAb were evaluated in vitro to determine binding affinity and immunoreactivity in HT29-MSLN and PDX (NCI-Meso16, NCI-Meso21) cells. For both the zirconium-89 conjugates, in vivo studies (biodistribution/imaging) were performed at days 1, 3, and 6, from which tissue uptake was determined. Results: Both the conjugates demonstrated a low nanomolar binding affinity for MSLN and >95% immunoreactivity. In all the three tumor types, biodistribution of [89Zr]Zr-DFO-MSLN-mAb resulted in higher tumor uptake(15.88-28-33%ID/g) at all time points compared with [89Zr]Zr-3,2-HOPO-MSLN-mAb(7-13.07%ID/g). [89Zr]Zr-3,2-HOPO-MSLN-mAb femur uptake was always higher than [89Zr]Zr-DFO-MSLN-mAb, and imaging results concurred with the biodistribution studies. Conclusions: Even though the conjugates exhibited a high binding affinity for MSLN, [89Zr]Zr-DFO-MSLN-mAb showed a higher tumor and lower femur uptake than [89Zr]Zr-3,2-HOPO-MSLN-mAb. Nevertheless, [89Zr]Zr-3,2-HOPO-MSLN-mAb could be used to study organ distribution and lesion uptake with the caveat of detecting MSLN-positive bone lesions. Clinical trial (NCT03507452).
Keywords: 32-HOPO; DFO; PET imaging; antibody conjugate; mesothelin; zirconium-89.
Conflict of interest statement
A.S.C. holds a patent on 3,2-HOPO-MSLN-mAb conjugate. Other authors declare that he/she has no potential conflict of interest.
Figures


Similar articles
-
Hydroxypyridinones as a Very Promising Platform for Targeted Diagnostic and Therapeutic Radiopharmaceuticals.Molecules. 2021 Nov 19;26(22):6997. doi: 10.3390/molecules26226997. Molecules. 2021. PMID: 34834087 Free PMC article. Review.
-
89Zr-3,2-HOPO-Mesothelin Antibody PET Imaging Reflects Tumor Uptake of Mesothelin-Targeted 227Th-Conjugate Therapy in Mice.J Nucl Med. 2022 Nov;63(11):1715-1721. doi: 10.2967/jnumed.121.263079. Epub 2022 Apr 14. J Nucl Med. 2022. PMID: 35422447
-
p-SCN-Bn-HOPO: A Superior Bifunctional Chelator for (89)Zr ImmunoPET.Bioconjug Chem. 2015 Dec 16;26(12):2579-91. doi: 10.1021/acs.bioconjchem.5b00572. Epub 2015 Nov 25. Bioconjug Chem. 2015. PMID: 26550847 Free PMC article.
-
Comparison of the octadentate bifunctional chelator DFO*-pPhe-NCS and the clinically used hexadentate bifunctional chelator DFO-pPhe-NCS for 89Zr-immuno-PET.Eur J Nucl Med Mol Imaging. 2017 Feb;44(2):286-295. doi: 10.1007/s00259-016-3499-x. Epub 2016 Aug 30. Eur J Nucl Med Mol Imaging. 2017. PMID: 27573793 Free PMC article.
-
89Zr-Immuno-Positron Emission Tomography in Oncology: State-of-the-Art 89Zr Radiochemistry.Bioconjug Chem. 2017 Sep 20;28(9):2211-2223. doi: 10.1021/acs.bioconjchem.7b00325. Epub 2017 Aug 24. Bioconjug Chem. 2017. PMID: 28767228 Free PMC article. Review.
Cited by
-
Preparation of a Zirconium-89 Labeled Clickable DOTA Complex and Its Antibody Conjugate.Pharmaceuticals (Basel). 2024 Apr 9;17(4):480. doi: 10.3390/ph17040480. Pharmaceuticals (Basel). 2024. PMID: 38675440 Free PMC article.
-
Evaluation of Candidate Theranostics for 227Th/89Zr Paired Radioimmunotherapy of Lymphoma.J Nucl Med. 2023 Jul;64(7):1062-1068. doi: 10.2967/jnumed.122.264979. Epub 2023 May 4. J Nucl Med. 2023. PMID: 37142300 Free PMC article.
-
Hydroxypyridinones as a Very Promising Platform for Targeted Diagnostic and Therapeutic Radiopharmaceuticals.Molecules. 2021 Nov 19;26(22):6997. doi: 10.3390/molecules26226997. Molecules. 2021. PMID: 34834087 Free PMC article. Review.
-
ImmunoPET for mesothelin positive tissues using bio-orthogonal in-vivo click chemistry.Nucl Med Biol. 2025 Jul 15;148-149:109051. doi: 10.1016/j.nucmedbio.2025.109051. Online ahead of print. Nucl Med Biol. 2025. PMID: 40694891
-
Toward Optimized 89Zr-Immuno-PET: Side-by-Side Comparison of [89Zr]Zr-DFO-, [89Zr]Zr-3,4,3-(LI-1,2-HOPO)- and [89Zr]Zr-DFO*-Cetuximab for Tumor Imaging: Which Chelator Is the Most Suitable?Pharmaceutics. 2022 Oct 4;14(10):2114. doi: 10.3390/pharmaceutics14102114. Pharmaceutics. 2022. PMID: 36297549 Free PMC article.
References
-
- Targeted Alpha Therapy Working G, Parker C, Lewington V, et al. . Targeted alpha therapy, an emerging class of cancer agents: A review. JAMA Oncol 2018;4:1765. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
