Comparative evaluation of a dual-target real-time RT-PCR assay for COVID-19 diagnosis and assessment of performance in pooled saliva and nasopharyngeal swab samples
- PMID: 34014785
- PMCID: PMC8182820
- DOI: 10.1080/14737159.2021.1933445
Comparative evaluation of a dual-target real-time RT-PCR assay for COVID-19 diagnosis and assessment of performance in pooled saliva and nasopharyngeal swab samples
Abstract
Objectives: Sensitive molecular diagnostic assays are essential for COVID-19 diagnosis. We evaluated the Hecin Scientific SARS-CoV-2 nucleic acid test kit, a dual-target real-time RT-PCR assay targeting the SARS-CoV-2 N and ORF1ab genes.
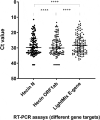
Methods: The Hecin test kit's diagnostic performance in detecting SARS-CoV-2 RNA was compared to the LightMix Modular SARS and Wuhan CoV E-gene kit (TIB Molbiol) and an in-house single-tube nested real-time RT-PCR using 296 clinical specimens, 11 proficiency testing samples, and 30 low-positive deep throat saliva and nasopharyngeal swab (NPS) samples pooled into negative samples in ratios of 1:5, 1:10, and 1:30.
Results: The limit-of-detection of the Hecin test kit was around 500 dC/mL for the N and ORF1ab targets. Sensitivity and specificity of the Hecin test kit were 98.1% (95% CI: 93.4-99.8%) and 100% (98.1-100%), respectively, when measured against the reference method. The Hecin test kit showed fair sensitivity (80%) in low-positive NPS samples pooled in ratios of 1:5 and 1:10. Its performance in pooled samples could be dramatically improved by adjusting the assay Ct cutoff.
Conclusion: The Hecin test kit enables sensitive and specific detection of SARS-CoV-2 in clinical samples and pooled samples.
Keywords: COVID-19; SARS-CoV-2; diagnostic test evaluation; pooling; real-time RT-PCR.
Figures

Similar articles
-
Development and Evaluation of AccuPower COVID-19 Multiplex Real-Time RT-PCR Kit and AccuPower SARS-CoV-2 Multiplex Real-Time RT-PCR Kit for SARS-CoV-2 Detection in Sputum, NPS/OPS, Saliva and Pooled Samples.PLoS One. 2022 Feb 10;17(2):e0263341. doi: 10.1371/journal.pone.0263341. eCollection 2022. PLoS One. 2022. PMID: 35143538 Free PMC article.
-
Clinical evaluation of a multiplex real-time RT-PCR assay for detection of SARS-CoV-2 in individual and pooled upper respiratory tract samples.Arch Virol. 2021 Sep;166(9):2551-2561. doi: 10.1007/s00705-021-05148-1. Epub 2021 Jul 14. Arch Virol. 2021. PMID: 34259914 Free PMC article.
-
Clinical Performance of Direct RT-PCR Testing of Raw Saliva for Detection of SARS-CoV-2 in Symptomatic and Asymptomatic Individuals.Microbiol Spectr. 2022 Dec 21;10(6):e0222922. doi: 10.1128/spectrum.02229-22. Epub 2022 Nov 21. Microbiol Spectr. 2022. PMID: 36409097 Free PMC article.
-
Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis.Lancet Infect Dis. 2021 Sep;21(9):1233-1245. doi: 10.1016/S1473-3099(21)00146-8. Epub 2021 Apr 12. Lancet Infect Dis. 2021. PMID: 33857405 Free PMC article.
-
Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: A systematic review and meta-analysis.J Med Virol. 2021 Feb;93(2):719-725. doi: 10.1002/jmv.26349. Epub 2020 Aug 2. J Med Virol. 2021. PMID: 32706393 Free PMC article.
Cited by
-
An improvement of current hypercube pooling PCR tests for SARS-CoV-2 detection.Front Public Health. 2022 Oct 19;10:994712. doi: 10.3389/fpubh.2022.994712. eCollection 2022. Front Public Health. 2022. PMID: 36339215 Free PMC article.
-
The best pooling strategy to reduce polymerase chain reaction tests during the coronavirus disease-19 pandemic at low prevalence.Tzu Chi Med J. 2025 May 23;37(3):299-303. doi: 10.4103/tcmj.tcmj_244_24. eCollection 2025 Jul-Sep. Tzu Chi Med J. 2025. PMID: 40741619 Free PMC article.
-
Performance evaluation of Biofire Film Array Respiratory Panel 2.1 for SARS-CoV-2 detection in a pediatric hospital setting.PLoS One. 2023 Oct 5;18(10):e0292314. doi: 10.1371/journal.pone.0292314. eCollection 2023. PLoS One. 2023. PMID: 37797063 Free PMC article.
-
The Diagnostic Performance of Various Clinical Specimens for the Detection of COVID-19: A Meta-Analysis of RT-PCR Studies.Diagnostics (Basel). 2023 Sep 26;13(19):3057. doi: 10.3390/diagnostics13193057. Diagnostics (Basel). 2023. PMID: 37835801 Free PMC article. Review.
-
Development and Validation of a Novel COVID-19 nsp8 One-Tube RT-LAMP-CRISPR Assay for SARS-CoV-2 Diagnosis.Microbiol Spectr. 2022 Dec 21;10(6):e0196222. doi: 10.1128/spectrum.01962-22. Epub 2022 Nov 29. Microbiol Spectr. 2022. PMID: 36445095 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
