ERAD-related E2 and E3 enzymes modulate the drought response by regulating the stability of PIP2 aquaporins
- PMID: 34015125
- PMCID: PMC8408458
- DOI: 10.1093/plcell/koab141
ERAD-related E2 and E3 enzymes modulate the drought response by regulating the stability of PIP2 aquaporins
Abstract
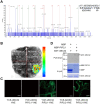
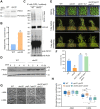
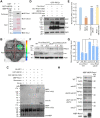
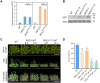
Endoplasmic reticulum-associated degradation (ERAD) is known to regulate plant responses to diverse stresses, yet its underlying molecular mechanisms and links to various stress signaling pathways are poorly understood. Here, we show that the ERAD component ubiquitin-conjugating enzyme UBC32 positively regulates drought tolerance in Arabidopsis thaliana by targeting the aquaporins PIP2;1 and PIP2;2 for degradation. Furthermore, we demonstrate that the RING-type ligase Rma1 acts together with UBC32 and that the E2 activity of UBC32 is essential for the ubiquitination of Rma1. This complex ubiquitinates a phosphorylated form of PIP2;1 at Lys276 to promote its degradation, thereby enhancing plant drought tolerance. Extending these molecular insights into crops, we show that overexpression of Arabidopsis UBC32 also improves drought tolerance in rice (Oryza sativa). Thus, beyond uncovering the molecular basis of an ERAD-regulated stress response, our study suggests multiple potential strategies for engineering crops with improved drought tolerance.
© American Society of Plant Biologists 2021. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Ahn MY, Oh TR, Seo DH, Kim JH, Cho NH, Kim WT (2018) Arabidopsis group XIV ubiquitin-conjugating enzymes AtUBC32, AtUBC33, and AtUBC34 play negative roles in drought stress response. J Plant Physiol 230: 73–79 - PubMed
-
- Alexandersson E, Fraysse L, Sjovall-Larsen S, Gustavsson S, Fellert M, Karlsson M, Johanson U, Kjellbom P (2005) Whole gene family expression and drought stress regulation of aquaporins. Plant Mol Biol 59: 469–484 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

