Deep Learning in Biomedical Optics
- PMID: 34015146
- PMCID: PMC8273152
- DOI: 10.1002/lsm.23414
Deep Learning in Biomedical Optics
Abstract
This article reviews deep learning applications in biomedical optics with a particular emphasis on image formation. The review is organized by imaging domains within biomedical optics and includes microscopy, fluorescence lifetime imaging, in vivo microscopy, widefield endoscopy, optical coherence tomography, photoacoustic imaging, diffuse tomography, and functional optical brain imaging. For each of these domains, we summarize how deep learning has been applied and highlight methods by which deep learning can enable new capabilities for optics in medicine. Challenges and opportunities to improve translation and adoption of deep learning in biomedical optics are also summarized. Lasers Surg. Med. © 2021 Wiley Periodicals LLC.
Keywords: biomedical optics; biophotonics; computer aided detection; deep learning; diffuse tomography; fluorescence lifetime; functional optical brain imaging; in vivo microscopy; machine learning; microscopy; optical coherence tomography; photoacoustic imaging; widefield endoscopy.
© 2021 Wiley Periodicals LLC.
Figures
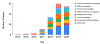



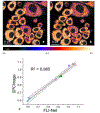


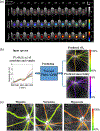




Comment in
-
Introduction to Special Biomedical Optical Imaging Issue.Lasers Surg Med. 2021 Aug;53(6):747. doi: 10.1002/lsm.23413. Lasers Surg Med. 2021. PMID: 34245596 No abstract available.
References
-
- Yann LeCun, Yoshua Bengio, Geoffrey Hinton. Deep learning nature. 2015;521:436–444. - PubMed
-
- Ian Goodfellow, Yoshua Bengio, Aaron Courville, Yoshua Bengio. Deep learning;1. MIT press Cambridge; 2016.
-
- Geert Litjens, Thijs Kooi, Ehteshami Bejnordi Babak, et al. A Survey on Deep Learning in Medical Image Analysis Medical Image Analysis. 2017;42:60–88. arXiv: 1702.05747. - PubMed
Publication types
MeSH terms
Grants and funding
- R21EB025621/NH/NIH HHS/United States
- R21 EB024700/EB/NIBIB NIH HHS/United States
- R21EB024700/NH/NIH HHS/United States
- R01 NS108464/NS/NINDS NIH HHS/United States
- R21 EB025621/EB/NIBIB NIH HHS/United States
- R01CA237267/NH/NIH HHS/United States
- R01EB019443/NH/NIH HHS/United States
- R01NS108464/NH/NIH HHS/United States
- R01CA250636/NH/NIH HHS/United States
- R01 CA237267/CA/NCI NIH HHS/United States
- R01 EY032163/EY/NEI NIH HHS/United States
- R01 CA250636/CA/NCI NIH HHS/United States
- R01CA207725/NH/NIH HHS/United States
- R21 EY029412/EY/NEI NIH HHS/United States
- R01 EB019443/EB/NIBIB NIH HHS/United States
- R01 CA207725/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

