The COVID-19 vaccine development: A pandemic paradigm
- PMID: 34015363
- PMCID: PMC8127526
- DOI: 10.1016/j.virusres.2021.198454
The COVID-19 vaccine development: A pandemic paradigm
Abstract
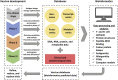
COVID-19 pandemic has resulted in millions of deaths and a social-economic crisis. A worldwide effort was made to develop efficient vaccines for this disease. A vaccine should produce immune responses with specific and neutralizing antibodies, and without harmful effects such as the antibody-dependent enhancement that may be associated with severe acute respiratory syndrome. Vaccine design involves the selection of platforms that includes viral, viral-vector, protein, nucleic acid, or trained immunity-based strategies. Its development initiates at a pre-clinical stage, followed by clinical trials when successful. Only if clinical trials show no significant evidence of safety concerns, vaccines can be manufactured, stored, and distributed to immunize the population. So far, regulatory authorities from many countries have approved nine vaccines with phase 3 results. In the current pandemic, a paradigm for the COVID-19 vaccine development has arisen, as many challenges must be overcome. Mass-production and cold-chain storage to immunize large human populations should be feasible and fast, and a combination of different vaccines may boost logistics and immunization. In silico trials is an emerging and innovative field that can be applied to predict and simulate immune, molecular, clinical, and epidemiological outcomes of vaccines to refine, reduce, and partially replace steps in vaccine development. Vaccine-resistant variants of SARS-CoV-2 might emerge, leading to the necessity of updates. A globally fair vaccine distribution system must prevail over vaccine nationalism for the world to return to its pre-pandemic status.
Keywords: Coronavirus; Emergency use; SARS-CoV-2; Vaccine safety; Vaccine strategies.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Agrawal A.S., Tao X., Algaissi A., Garron T., Narayanan K., Peng B.H., Couch R.B., Tseng C.T.K. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum. Vaccines Immunother. 2016;12:2351–2356. doi: 10.1080/21645515.2016.1177688. - DOI - PMC - PubMed
-
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. - DOI - PMC - PubMed
-
- Bartsch S.M., O’Shea K.J., Ferguson M.C., Bottazzi M.E., Wedlock P.T., Strych U., McKinnell J.A., Siegmund S.S., Cox S.N., Hotez P.J., Lee B.Y. Vaccine efficacy needed for a COVID-19 coronavirus vaccine to prevent or stop an epidemic as the sole intervention. Am. J. Prev. Med. 2020;59(4):493–503. doi: 10.1016/j.amepre.2020.06.011. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

