Real-world clinical performance of commercial SARS-CoV-2 rapid antigen tests in suspected COVID-19: A systematic meta-analysis of available data as of November 20, 2020
- PMID: 34015523
- PMCID: PMC8127520
- DOI: 10.1016/j.ijid.2021.05.029
Real-world clinical performance of commercial SARS-CoV-2 rapid antigen tests in suspected COVID-19: A systematic meta-analysis of available data as of November 20, 2020
Abstract
Objectives: Rapid antigen tests, or RATs, are a type of lateral flow chromatographic immunoassay utilized to aid the diagnosis of SARS-CoV-2 infection. We performed a systematic meta-analysis to compare the real-world performance of commercially available RATs.
Methods: We searched several databases and websites for manufacturer-independent prospective clinical performance studies comparing SARS-CoV-2 RATs and RT-PCR. Only studies on RATs that did not need a separate reader for result retrieval and that reported data on viral load, patients' symptom status, sample type, and PCR assay used were included.
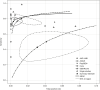
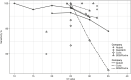
Results: 19 studies utilizing 11,109 samples with 2,509 RT-PCR-positives were included. RAT sensitivity varied between 28.9% (95% CI 16.4-44.3) and 98.3% (95% CI 91.1-99.7), likely dependent upon population characteristics, viral load, and symptom status. RAT specificity varied between 92.4% (95% CI 87.4-95.9) and 100% (95% CI 99.7-100) with one outlier. The RATs by Roche Diagnostics/SD Biosensor and Abbott had the highest pooled sensitivity (82.4% [95% CI 74.2-88.4] and 76.9% [95% CI 72.1-81.2], respectively). Sensitivity in high-viral-load samples (cycle threshold ≤25) showed heterogeneity among the different RATs.
Conclusion: The RATs offered by Roche Diagnostics/SD Biosensor and Abbott provide sufficient manufacturer-independent, real-world performance data to support their use to detect current SARS-CoV-2 infection, particularly in high-viral-load populations.
Keywords: COVID-19; Immunoassay; Lateral flow; Point-of-care testing; Rapid antigen test; SARS-CoV-2.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures



References
-
- Albert E., Torres I., Bueno F., Huntley D., Molla E., Fernández-Fuentes M., et al. Field evaluation of a rapid antigen test (PanbioTM COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centres. Clin Microbiol Infect. 2020;(November) doi: 10.1016/j.cmi.2020.11.004. S1198-1743X(1120)30697-30692. - DOI - PMC - PubMed
-
- Berger A., Ngo Nsoga M.T., Perez-Rodriguez F.J., Aad Y.A., Sattonnet-Roche P., Gayet-Ageron A., et al. Diagnostic accuracy of two commercial SARS-CoV-2 antigen-detecting rapid tests at the point of care in community-based testing centers. medRxiv. 2020 doi: 10.1101/2020.11.20.20235341. 11.20.20235341. - DOI - PMC - PubMed
-
- Bulilete O., Lorente P., Leiva A., Carandell E., Oliver A., Rojo E., et al. Evaluation of the PanbioTM rapid antigen test for SARS-CoV-2 in primary health care centers and test sites. medRxiv. 2020 doi: 10.1101/2020.11.13.20231316. 11.13.20231316. - DOI
-
- Centers for Disease Control and Prevention . 2020. Interim guidance for antigen testing for SARS-CoV-2.https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-gu... [Accessed 8 January 2021]
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

