Efficacy and Safety of Azithromycin for the Treatment of COVID-19: A Systematic Review and Meta-analysis
- PMID: 34015868
- PMCID: PMC8497767
- DOI: 10.4046/trd.2021.0075
Efficacy and Safety of Azithromycin for the Treatment of COVID-19: A Systematic Review and Meta-analysis
Abstract
Background: The lack of effective medications for coronavirus disease 2019 (COVID-19) has led to a trend of drug repurposing such as the case of azithromycin which shows immunomodulatory and anti-viral effect. Several clinical trials have shown conflicting results. It is currently unclear whether the available evidence is in favor or against the use of azithromycin in COVID-19 patients. Thus, the aim of this study was to investigate the efficacy and safety of azithromycin in COVID-19 patients.
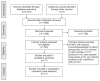
Methods: Four independent reviewers selected relevant studies from PubMed, ScienceDirect, EBSCO, and ProQuest published prior to March 2021. The protocol used in this study has been registered in PROSPERO (CRD42020224967).
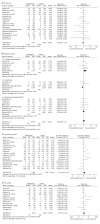
Results: We included 17 studies and found that the mortality rate (odds ratio [OR], 0.95; 95% confidence interval [CI], 0.76-1.19), need of respiratory support (OR, 1.30; 95% CI, 0.98-1.73), hospitalization rate (standardized mean difference, 0.12; 95% CI, -0.02 to 0.27), and intensive care unit transfer (OR, 1.21; 95% CI, 0.79-1.86) of azithromycin-treated group did not differ significantly (p>0.05) from those of the control group. Azithromycin treatment did not significantly increase the risk of getting secondary infection (OR, 1.23; 95% CI, 0.83-1.82), hypoglycemia (OR, 0.73; 95% CI, 0.38-1.40), gastrointestinal problems (OR, 1.03; 95% CI, 0.73-1.45) or electrocardiogram abnormalities (OR, 1.16; 95% CI, 0.94-1.42). The overall quality of evidence ranged from low to very low.
Conclusion: Azithromycin did not result in a superior clinical improvement in COVID-19 patients, although it was well-tolerated and safe to use.
Keywords: Azithromycin; COVID-19; Meta-analysis; Systematic Review; Treatment.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures




References
-
- WHO Coronavirus (COVID-19) Dashboard [Internet] Geneva: World Health Organization; 2021. [cited 2021 Apr 16]. Available from: https://covid19.who.int/
-
- Zarogoulidis P, Papanas N, Kioumis I, Chatzaki E, Maltezos E, Zarogoulidis K. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur J Clin Pharmacol. 2012;68:479–503. - PubMed
-
- Lee N, Wong CK, Chan MC, Yeung ES, Tam WW, Tsang OT, et al. Anti-inflammatory effects of adjunctive macrolide treatment in adults hospitalized with influenza: a randomized controlled trial. Antiviral Res. 2017;144:48–56. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

