GSDMD-Mediated Cardiomyocyte Pyroptosis Promotes Myocardial I/R Injury
- PMID: 34015941
- PMCID: PMC8291144
- DOI: 10.1161/CIRCRESAHA.120.318629
GSDMD-Mediated Cardiomyocyte Pyroptosis Promotes Myocardial I/R Injury
Abstract
[Figure: see text].
Keywords: caspase; cell death; inflammation; necrosis; pyroptosis.
Figures


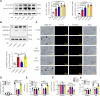
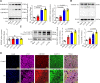
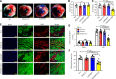

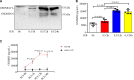
Comment in
-
Multiple Cell Death Programs Contribute to Myocardial Infarction.Circ Res. 2021 Jul 23;129(3):397-399. doi: 10.1161/CIRCRESAHA.121.319584. Epub 2021 Jul 22. Circ Res. 2021. PMID: 34292784 No abstract available.
References
-
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. ; CF/AHA Task Force. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013; 127:529–555. doi: 10.1161/CIR.0b013e3182742c84 - PubMed
-
- Ibáñez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015; 65:1454–1471. doi: 10.1016/j.jacc.2015.02.032 - PubMed
-
- Hillis LD, Lange RA. Myocardial infarction and the open-artery hypothesis. N Engl J Med. 2006; 355:2475–2477. doi: 10.1056/NEJMe068251 - PubMed
-
- Piper HM, García-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998; 38:291–300. doi: 10.1016/s0008-6363(98)00033-9 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

