The repurposed use of anesthesia machines to ventilate critically ill patients with coronavirus disease 2019 (COVID-19)
- PMID: 34016056
- PMCID: PMC8134805
- DOI: 10.1186/s12871-021-01376-9
The repurposed use of anesthesia machines to ventilate critically ill patients with coronavirus disease 2019 (COVID-19)
Abstract
Background: The surge of critically ill patients due to the coronavirus disease-2019 (COVID-19) overwhelmed critical care capacity in areas of northern Italy. Anesthesia machines have been used as alternatives to traditional ICU mechanical ventilators. However, the outcomes for patients with COVID-19 respiratory failure cared for with Anesthesia Machines is currently unknow. We hypothesized that COVID-19 patients receiving care with Anesthesia Machines would have worse outcomes compared to standard practice.
Methods: We designed a retrospective study of patients admitted with a confirmed COVID-19 diagnosis at a large tertiary urban hospital in northern Italy. Two care units were included: a 27-bed standard ICU and a 15-bed temporary unit emergently opened in an operating room setting. Intubated patients assigned to Anesthesia Machines (AM group) were compared to a control cohort treated with standard mechanical ventilators (ICU-VENT group). Outcomes were assessed at 60-day follow-up. A multivariable Cox regression analysis of risk factors between survivors and non-survivors was conducted to determine the adjusted risk of death for patients assigned to AM group.
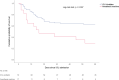
Results: Complete daily data from 89 mechanically ventilated patients consecutively admitted to the two units were analyzed. Seventeen patients were included in the AM group, whereas 72 were in the ICU-VENT group. Disease severity and intensity of treatment were comparable between the two groups. The 60-day mortality was significantly higher in the AM group compared to the ICU-vent group (12/17 vs. 27/72, 70.6% vs. 37.5%, respectively, p = 0.016). Allocation to AM group was associated with a significantly increased risk of death after adjusting for covariates (HR 4.05, 95% CI: 1.75-9.33, p = 0.001). Several incidents and complications were reported with Anesthesia Machine care, raising safety concerns.
Conclusions: Our results support the hypothesis that care associated with the use of Anesthesia Machines is inadequate to provide long-term critical care to patients with COVID-19. Added safety risks must be considered if no other option is available to treat severely ill patients during the ongoing pandemic.
Clinical trial number: Not applicable.
Keywords: ARDS; Anesthesia machine; COVID-19; Intensive care unit; Mechanical ventilation.
Conflict of interest statement
AM reports receiving statistical consulting fees from the University of Chicago. RDU reports unrelated fees or research funding from Merck, Covidien/Medtronic, AcelRx, Acacia Pharma, Takeda, and federal funding by the NIH NIH/NIDA - 1R34DA048268-01A1, AHRQ R01 HS025718-01A1, NSF Award #1838796.
LB receives salary support from K23 HL128882/NHLBI NIH as principal investigator for his work on hemolysis and nitric oxide. LB receives technologies and devices from iNO Therapeutics LLC, Praxair Inc., Masimo Corp. LB receives a grant from iNO Therapeutics LLC. The other authors declare no competing interests.
Figures
Update of
-
The repurposed use of anesthesia machines to ventilate critically ill patients with Coronavirus Disease 2019 (COVID-19).Res Sq [Preprint]. 2021 Feb 12:rs.3.rs-228821. doi: 10.21203/rs.3.rs-228821/v1. Res Sq. 2021. Update in: BMC Anesthesiol. 2021 May 20;21(1):155. doi: 10.1186/s12871-021-01376-9. PMID: 33594358 Free PMC article. Updated. Preprint.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical