Anastrozole and levonorgrestrel-releasing intrauterine device in the treatment of endometriosis: a randomized clinical trial
- PMID: 34016111
- PMCID: PMC8138989
- DOI: 10.1186/s12905-021-01347-9
Anastrozole and levonorgrestrel-releasing intrauterine device in the treatment of endometriosis: a randomized clinical trial
Abstract
Background: To study the effectiveness of an aromatase inhibitor (Anastrozole) associated with levonorgestrel-releasing intrauterine device (LNG-IUD, Mirena®) in the treatment of endometriosis.
Methods: Prospective, randomized clinical trial.
Setting: University Hospital (single center). Elegibility criteria: Endometriomas > 3 × 4 cm, CA-125 > 35 U/mL and endometriosis symptoms.
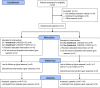
Patients: Thirty-one women randomized to anastrozole + Mirena® + Conservative Surgery(CS) (n = 8), anastrozole + Mirena® + transvaginal ultrasound-guided puncture-aspiration (TUGPA) (n = 7), Mirena® + CS (n = 9), or Mirena® + TUGPA (n = 7).
Interventions: Anastrozole 1 mg/day and/or only Mirena® for 6 months; CS (ovarian and fertility-sparing) or TUGPA of endometriomas one month after starting medical treatment.
Main outcome measures: Visual analogic scale for symptoms, CA-125 levels, ultrasound findings of endometriomas and recurrences.
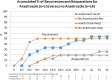
Results: A significant improvement in symptoms during the treatment (difference of 43%, 95% CI 29.9-56.2) occurred, which was maintained at 1 and 2 years. It was more significant in patients including anastrozole in their treatment (51%, 95% CI 33.3-68.7). For CA-125, the most significant decrease was observed in patients not taking anastrozole (73.8%, 95% CI 64.2-83.4 vs. 53.8%, 95% CI 25.7-81.6 under Mirena® + anastrozole). After CS for endometriosis, a reduction of ultrasound findings of endometriomas and long-term recurrence occurred, with or without anastrozole. At 4.2 ± 1.7 years (95% CI 3.57-4.85), 88% of the patients who underwent CS were asymptomatic, without medication or reoperation, compared to only 21% if TUGPA was performed, with or without anastrozole (p = 0.019).
Conclusions: Dosing anastrozole for 6 months, starting one month before CS of endometriosis, reduces significantly the painful symptoms and delays recurrence, but has no other significant advantages over the single insertion of LNG-IUD (Mirena®) during the same time. Anastrozole and/or only Mirena® associated with TUGPA are not effective.
Trial registration: Eudra CT System of the European Medicines Agency (London, 29-Sept-2008) Nº EudraCT: 2008-005744-17 (07/11/2008). Date of enrolment of first patient: 15/01/2009.
Keywords: Anastrozole; Aromatase inhibitors; Clinical trial; Endometriomas; Endometriosis; Levonorgestrel-IUD.
Conflict of interest statement
We declare no competing interests. Not applicable.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

