"Real-world" radiomics from multi-vendor MRI: an original retrospective study on the prediction of nodal status and disease survival in breast cancer, as an exemplar to promote discussion of the wider issues
- PMID: 34016188
- PMCID: PMC8136229
- DOI: 10.1186/s40644-021-00406-6
"Real-world" radiomics from multi-vendor MRI: an original retrospective study on the prediction of nodal status and disease survival in breast cancer, as an exemplar to promote discussion of the wider issues
Abstract
Background: Most MRI radiomics studies to date, even multi-centre ones, have used "pure" datasets deliberately accrued from single-vendor, single-field-strength scanners. This does not reflect aspirations for the ultimate generalisability of AI models. We therefore investigated the development of a radiomics signature from heterogeneous data originating on six different imaging platforms, for a breast cancer exemplar, in order to provide input into future discussions of the viability of radiomics in "real-world" scenarios where image data are not controlled by specific trial protocols but reflective of routine clinical practice.
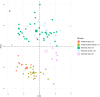
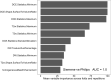
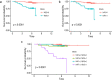
Methods: One hundred fifty-six patients with pathologically proven breast cancer underwent multi-contrast MRI prior to neoadjuvant chemotherapy and/or surgery. From these, 92 patients were identified for whom T2-weighted, diffusion-weighted and contrast-enhanced T1-weighted sequences were available, as well as key clinicopathological variables. Regions-of-interest were drawn on the above image types and, from these, semantic and calculated radiomics features were derived. Classification models using a variety of methods, both with and without recursive feature elimination, were developed to predict pathological nodal status. Separately, we applied the same methods to analyse the information carried by the radiomic features regarding the originating scanner type and field strength. Repeated, ten-fold cross-validation was employed to verify the results. In parallel work, survival modelling was performed using random survival forests.
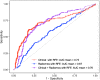
Results: Prediction of nodal status yielded mean cross-validated AUC values of 0.735 ± 0.15 (SD) for clinical variables alone, 0.673 ± 0.16 (SD) for radiomic features only, and 0.764 ± 0.16 (SD) for radiomics and clinical features together. Prediction of scanner platform from the radiomics features yielded extremely high values of AUC between 0.91 and 1 for the different classes examined indicating the presence of confounding features for the nodal status classification task. Survival analysis, gave out-of-bag prediction errors of 19.3% (clinical features only), 36.9-51.8% (radiomic features from different combinations of image contrasts), and 26.7-35.6% (clinical plus radiomics features).
Conclusions: Radiomic classification models whose predictive ability was consistent with previous single-vendor, single-field strength studies have been obtained from multi-vendor, multi-field-strength data, despite clear confounding information being present. However, our sample size was too small to obtain useful survival modelling results.
Keywords: Feature reduction; Multi-vendor; Nodal status; Radiomics; Survival.
Conflict of interest statement
The authors declare that they have no competing interests.
Nicholas Turner has received advisory board honoraria from Astra Zeneca, Bristol-Myers Squibb, Lilly, Merck Sharpe and Dohme, Novartis, Pfizer, Roche/Genentech, Bicycle Therapeutics, Taiho, Zeno pharmaceuticals, Repare therapeutics and research funding from Astra Zeneca, BioRad, Pfizer, Roche/Genentech, Clovis, Merck Sharpe and Dohme, and Guardant Health.
Figures





Similar articles
-
Magnetic resonance imaging radiomics predicts preoperative axillary lymph node metastasis to support surgical decisions and is associated with tumor microenvironment in invasive breast cancer: A machine learning, multicenter study.EBioMedicine. 2021 Jul;69:103460. doi: 10.1016/j.ebiom.2021.103460. Epub 2021 Jul 4. EBioMedicine. 2021. PMID: 34233259 Free PMC article. Clinical Trial.
-
Preoperative prediction of lymphovascular invasion in invasive breast cancer with dynamic contrast-enhanced-MRI-based radiomics.J Magn Reson Imaging. 2019 Sep;50(3):847-857. doi: 10.1002/jmri.26688. Epub 2019 Feb 17. J Magn Reson Imaging. 2019. PMID: 30773770
-
Impact of Machine Learning With Multiparametric Magnetic Resonance Imaging of the Breast for Early Prediction of Response to Neoadjuvant Chemotherapy and Survival Outcomes in Breast Cancer Patients.Invest Radiol. 2019 Feb;54(2):110-117. doi: 10.1097/RLI.0000000000000518. Invest Radiol. 2019. PMID: 30358693 Free PMC article.
-
Exploring breast cancer response prediction to neoadjuvant systemic therapy using MRI-based radiomics: A systematic review.Eur J Radiol. 2019 Dec;121:108736. doi: 10.1016/j.ejrad.2019.108736. Epub 2019 Nov 6. Eur J Radiol. 2019. PMID: 31734639
-
MRI radiomics in the prediction of therapeutic response to neoadjuvant therapy for locoregionally advanced rectal cancer: a systematic review.Expert Rev Anticancer Ther. 2021 Apr;21(4):425-449. doi: 10.1080/14737140.2021.1860762. Epub 2021 Jan 11. Expert Rev Anticancer Ther. 2021. PMID: 33289435
Cited by
-
A Low-Dose CT-Based Radiomic Model to Improve Characterization and Screening Recall Intervals of Indeterminate Prevalent Pulmonary Nodules.Diagnostics (Basel). 2021 Sep 3;11(9):1610. doi: 10.3390/diagnostics11091610. Diagnostics (Basel). 2021. PMID: 34573951 Free PMC article.
-
Prediction of Breast Cancer Response to Neoadjuvant Therapy with Machine Learning: A Clinical, MRI-Qualitative, and Radiomics Approach.Life (Basel). 2025 Jul 23;15(8):1165. doi: 10.3390/life15081165. Life (Basel). 2025. PMID: 40868813 Free PMC article.
-
Characterization of Breast Tumors from MR Images Using Radiomics and Machine Learning Approaches.J Pers Med. 2023 Jun 28;13(7):1062. doi: 10.3390/jpm13071062. J Pers Med. 2023. PMID: 37511674 Free PMC article.
-
Successes and challenges in extracting information from DICOM image databases for audit and research.Br J Radiol. 2023 Nov;96(1151):20230104. doi: 10.1259/bjr.20230104. Epub 2023 Sep 12. Br J Radiol. 2023. PMID: 37698251 Free PMC article. Review.
-
Prediction of Incomplete Response of Primary Tumour Based on Clinical and Radiomics Features in Inoperable Head and Neck Cancers after Definitive Treatment.J Pers Med. 2022 Jun 30;12(7):1092. doi: 10.3390/jpm12071092. J Pers Med. 2022. PMID: 35887587 Free PMC article.
References
-
- Zwanenburg A, Vallières M, Abdalah MA, Aerts HJWL, Andrearczyk V, Apte A, Ashrafinia S, Bakas S, Beukinga RJ, Boellaard R, Bogowicz M, Boldrini L, Buvat I, Cook GJR, Davatzikos C, Depeursinge A, Desseroit MC, Dinapoli N, Dinh CV, Echegaray S, el Naqa I, Fedorov AY, Gatta R, Gillies RJ, Goh V, Götz M, Guckenberger M, Ha SM, Hatt M, Isensee F, Lambin P, Leger S, Leijenaar RTH, Lenkowicz J, Lippert F, Losnegård A, Maier-Hein KH, Morin O, Müller H, Napel S, Nioche C, Orlhac F, Pati S, Pfaehler EAG, Rahmim A, Rao AUK, Scherer J, Siddique MM, Sijtsema NM, Socarras Fernandez J, Spezi E, Steenbakkers RJHM, Tanadini-Lang S, Thorwarth D, Troost EGC, Upadhaya T, Valentini V, van Dijk LV, van Griethuysen J, van Velden FHP, Whybra P, Richter C, Löck S. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. 2020;295(2):328–338. doi: 10.1148/radiol.2020191145. - DOI - PMC - PubMed
-
- Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, Forster K, Aerts HJWL, Dekker A, Fenstermacher D, Goldgof DB, Hall LO, Lambin P, Balagurunathan Y, Gatenby RA, Gillies RJ. Radiomics: the process and the challenges. Magn Reson Imaging. 2012;30(9):1234–1248. doi: 10.1016/j.mri.2012.06.010. - DOI - PMC - PubMed
-
- Mes SW, van Velden FHP, Peltenburg B, Peeters CFW, te Beest DE, van de Wiel MA, Mekke J, Mulder DC, Martens RM, Castelijns JA, Pameijer FA, de Bree R, Boellaard R, Leemans CR, Brakenhoff RH, de Graaf P. Outcome prediction of head and neck squamous cell carcinoma by MRI radiomic signatures. Eur Radiol. 2020;30(11):6311–6321. doi: 10.1007/s00330-020-06962-y. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

