Chitinase-Like Protein Ym2 (Chil4) Regulates Regeneration of the Olfactory Epithelium via Interaction with Inflammation
- PMID: 34016714
- PMCID: PMC8244980
- DOI: 10.1523/JNEUROSCI.1601-20.2021
Chitinase-Like Protein Ym2 (Chil4) Regulates Regeneration of the Olfactory Epithelium via Interaction with Inflammation
Abstract
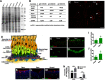
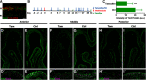
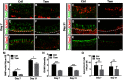
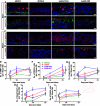
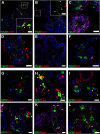
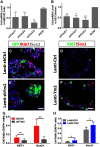
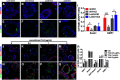
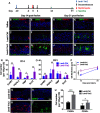
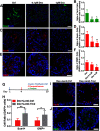
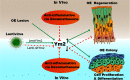
The adult olfactory epithelium (OE) regenerates sensory neurons and nonsensory supporting cells from resident stem cells after injury. How supporting cells contribute to OE regeneration remains largely unknown. In this study, we elucidated a novel role of Ym2 (also known as Chil4 or Chi3l4), a chitinase-like protein expressed in supporting cells, in regulating regeneration of the injured OE in vivo in both male and female mice and cell proliferation/differentiation in OE colonies in vitro We found that Ym2 expression was enhanced in supporting cells after OE injury. Genetic knockdown of Ym2 in supporting cells attenuated recovery of the injured OE, while Ym2 overexpression by lentiviral infection accelerated OE regeneration. Similarly, Ym2 bidirectionally regulated cell proliferation and differentiation in OE colonies. Furthermore, anti-inflammatory treatment reduced Ym2 expression and delayed OE regeneration in vivo and cell proliferation/differentiation in vitro, which were counteracted by Ym2 overexpression. Collectively, this study revealed a novel role of Ym2 in OE regeneration and cell proliferation/differentiation of OE colonies via interaction with inflammatory responses, providing new clues to the function of supporting cells in these processes.SIGNIFICANCE STATEMENT The mammalian olfactory epithelium (OE) is a unique neural tissue that regenerates sensory neurons and nonsensory supporting cells throughout life and postinjury. How supporting cells contribute to this process is not entirely understood. Here we report that OE injury causes upregulation of a chitinase-like protein, Ym2, in supporting cells, which facilitates OE regeneration. Moreover, anti-inflammatory treatment reduces Ym2 expression and delays OE regeneration, which are counteracted by Ym2 overexpression. This study reveals an important role of supporting cells in OE regeneration and provides a critical link between Ym2 and inflammation in this process.
Keywords: Ym2; chitinase-like protein; inflammation; olfactory epithelium; regeneration; supporting cell.
Copyright © 2021 the authors.
Figures




Similar articles
-
Deficiency of Aging-Related Gene Chitinase-Like 4 Impairs Olfactory Epithelium Homeostasis.Cell Prolif. 2025 Aug;58(8):e70055. doi: 10.1111/cpr.70055. Epub 2025 May 19. Cell Prolif. 2025. PMID: 40389328 Free PMC article.
-
[Chitinase-like protein Ym2 regulates olfactory epithelium homeostasis and olfactory behavior in mice].Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2025 Feb 7;60(2):134-143. doi: 10.3760/cma.j.cn115330-20240423-00226. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2025. PMID: 39979086 Chinese.
-
Primary Cilia on Horizontal Basal Cells Regulate Regeneration of the Olfactory Epithelium.J Neurosci. 2015 Oct 7;35(40):13761-72. doi: 10.1523/JNEUROSCI.1708-15.2015. J Neurosci. 2015. PMID: 26446227 Free PMC article.
-
Transcriptional regulatory network during development in the olfactory epithelium.BMB Rep. 2015 Nov;48(11):599-608. doi: 10.5483/bmbrep.2015.48.11.177. BMB Rep. 2015. PMID: 26303973 Free PMC article. Review.
-
Transcriptional and Epigenetic Control of Mammalian Olfactory Epithelium Development.Mol Neurobiol. 2018 Nov;55(11):8306-8327. doi: 10.1007/s12035-018-0987-y. Epub 2018 Mar 12. Mol Neurobiol. 2018. PMID: 29532253 Review.
Cited by
-
Analysis of lncRNA and mRNA Repertoires in Lung From BAFF-R-Deficient Pneumocystis-Infected Mice.Front Immunol. 2022 Jun 10;13:898660. doi: 10.3389/fimmu.2022.898660. eCollection 2022. Front Immunol. 2022. PMID: 35774783 Free PMC article.
-
Deficiency of Aging-Related Gene Chitinase-Like 4 Impairs Olfactory Epithelium Homeostasis.Cell Prolif. 2025 Aug;58(8):e70055. doi: 10.1111/cpr.70055. Epub 2025 May 19. Cell Prolif. 2025. PMID: 40389328 Free PMC article.
-
The IL-4-IL-4Rα axis modulates olfactory neuroimmune signaling to induce loss of smell.Allergy. 2025 Feb;80(2):440-461. doi: 10.1111/all.16338. Epub 2024 Oct 17. Allergy. 2025. PMID: 39418114 Free PMC article.
-
Horizontal basal cells self-govern their neurogenic potential during injury-induced regeneration of the olfactory epithelium.Development. 2023 Jun 15;150(12):dev201552. doi: 10.1242/dev.201552. Epub 2023 Jun 26. Development. 2023. PMID: 37260223 Free PMC article.
-
Lgr5+ cells are required and dynamically participate in olfactory epithelium regeneration: a revisiting shows Lgr5 expression in multiple cell lineages.Theranostics. 2022 Jul 18;12(13):5631-5644. doi: 10.7150/thno.60636. eCollection 2022. Theranostics. 2022. PMID: 35966594 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
