Peptide: MHC-based DNA vaccination strategy to activate natural killer cells by targeting killer cell immunoglobulin-like receptors
- PMID: 34016721
- PMCID: PMC8141441
- DOI: 10.1136/jitc-2020-001912
Peptide: MHC-based DNA vaccination strategy to activate natural killer cells by targeting killer cell immunoglobulin-like receptors
Abstract
Background: Natural killer (NK) cells are increasingly being recognized as agents for cancer immunotherapy. The killer cell immunoglobulin-like receptors (KIRs) are expressed by NK cells and are immunogenetic determinants of the outcome of cancer. In particular, KIR2DS2 is associated with protective responses to several cancers and also direct recognition of cancer targets in vitro. Due to the high homology between activating and inhibitory KIR genes to date, it has been challenging to target individual KIR for therapeutic benefit.
Methods: A novel KIR2DS2-targeting therapeutic peptide:MHC DNA vaccine was designed and used to immunize mice transgenic for KIR genes (KIR-Tg). NK cells were isolated from the livers and spleens of vaccinated mice and then analyzed for activation by flow cytometry, RNA profiling and cytotoxicity assays. In vivo assays of NK cell function using a syngeneic cancer model (B16 melanoma) and an adoptive transfer model for human hepatocellular carcinoma (Huh7) were performed.
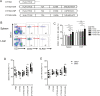
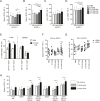
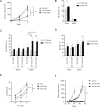
Results: Injecting KIR-Tg mice with the vaccine construct activated NK cells in both liver and spleens of mice, with preferential activation of KIR2DS2-positive NK cells. KIR-specific activation was most marked on the CD11b+CD27+ mature subset of NK cells. RNA profiling indicated that the DNA vaccine upregulated genes associated with cellular metabolism and downregulated genes related to histone H3 methylation, which are associated with immune cell maturation and NK cell function. Vaccination led to canonical and cross-reactive peptide:MHC-specific NK cell responses. In vivo, DNA vaccination led to enhanced antitumor responses against B16F10 melanoma cells and also enhanced responses against a tumor model expressing the KIR2DS2 ligand HLA-C*0102.
Conclusion: We show the feasibility of a peptide-based KIR-targeting vaccine strategy to activate NK cells and hence generate functional antitumor responses. This approach does not require detailed knowledge of the tumor peptidomes nor HLA matching with the patient. It therefore offers a novel opportunity for targeting NK cells for cancer immunotherapy.
Keywords: immunity; immunogenicity; innate; killer cells; natural; vaccine.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: The University of Southampton has applied for patents associated with the vaccine constructs described in this work.
Figures

Similar articles
-
Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands.J Immunol. 2007 Nov 1;179(9):5977-89. doi: 10.4049/jimmunol.179.9.5977. J Immunol. 2007. PMID: 17947671
-
Natural killer-cell immunoglobulin-like receptors trigger differences in immune response to SARS-CoV-2 infection.PLoS One. 2021 Aug 5;16(8):e0255608. doi: 10.1371/journal.pone.0255608. eCollection 2021. PLoS One. 2021. PMID: 34352002 Free PMC article.
-
Genetic polymorphism of NK receptors and their ligands in melanoma patients: prevalence of inhibitory over activating signals.Cancer Immunol Immunother. 2005 Feb;54(2):172-8. doi: 10.1007/s00262-004-0575-z. Epub 2004 Jul 10. Cancer Immunol Immunother. 2005. PMID: 15248031 Free PMC article.
-
Impact of KIR/HLA ligand combinations on immune responses in malignant melanoma.Cancer Immunol Immunother. 2007 Jan;56(1):95-100. doi: 10.1007/s00262-006-0151-9. Epub 2006 Mar 18. Cancer Immunol Immunother. 2007. PMID: 16547704 Free PMC article. Review.
-
Regulation of NK cell responsiveness to achieve self-tolerance and maximal responses to diseased target cells.Immunol Rev. 2008 Aug;224:85-97. doi: 10.1111/j.1600-065X.2008.00658.x. Immunol Rev. 2008. PMID: 18759922 Free PMC article. Review.
Cited by
-
Peanut oral immunotherapy: current trends in clinical trials.Immunother Adv. 2022 Jan 31;2(1):ltac004. doi: 10.1093/immadv/ltac004. eCollection 2022. Immunother Adv. 2022. PMID: 35919493 Free PMC article. Review.
-
Relevance of Polymorphic KIR and HLA Class I Genes in NK-Cell-Based Immunotherapies for Adult Leukemic Patients.Cancers (Basel). 2021 Jul 27;13(15):3767. doi: 10.3390/cancers13153767. Cancers (Basel). 2021. PMID: 34359667 Free PMC article. Review.
-
Beyond CAR-T: The rise of CAR-NK cell therapy in asthma immunotherapy.J Transl Med. 2024 Aug 5;22(1):736. doi: 10.1186/s12967-024-05534-8. J Transl Med. 2024. PMID: 39103889 Free PMC article. Review.
-
Combination of Ablation and Immunotherapy for Hepatocellular Carcinoma: Where We Are and Where to Go.Front Immunol. 2021 Dec 15;12:792781. doi: 10.3389/fimmu.2021.792781. eCollection 2021. Front Immunol. 2021. PMID: 34975896 Free PMC article. Review.
-
The nuclear export protein XPO1 provides a peptide ligand for natural killer cells.Sci Adv. 2024 Aug 23;10(34):eado6566. doi: 10.1126/sciadv.ado6566. Epub 2024 Aug 23. Sci Adv. 2024. PMID: 39178254 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
