Modulation of lactate-lysosome axis in dendritic cells by clotrimazole potentiates antitumor immunity
- PMID: 34016722
- PMCID: PMC8141455
- DOI: 10.1136/jitc-2020-002155
Modulation of lactate-lysosome axis in dendritic cells by clotrimazole potentiates antitumor immunity
Abstract
Background: Dendritic cells (DCs) play a critical role in antitumor immunity, but the therapeutic efficacy of DC-mediated cancer vaccine remains low, partly due to unsustainable DC function in tumor antigen presentation. Thus, identifying drugs that could enhance DC-based antitumor immunity and uncovering the underlying mechanism may provide new therapeutic options for cancer immunotherapy.
Methods: In vitro antigen presentation assay was used for DC-modulating drug screening. The function of DC and T cells was measured by flow cytometry, ELISA, or qPCR. B16, MC38, CT26 tumor models and C57BL/6, Balb/c, nude, and Batf3-/- mice were used to analyze the in vivo therapy efficacy and impact on tumor immune microenvironment by clotrimazole treatment.
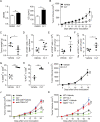
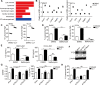
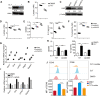
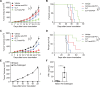
Results: By screening a group of small molecule inhibitors and the US Food and Drug Administration (FDA)-approved drugs, we identified that clotrimazole, an antifungal drug, could promote DC-mediated antigen presentation and enhance T cell response. Mechanistically, clotrimazole acted on hexokinase 2 to regulate lactate metabolic production and enhanced the lysosome pathway and Chop expression in DCs subsequently induced DC maturation and T cell activation. Importantly, in vivo clotrimazole administration induced intratumor immune infiltration and inhibited tumor growth depending on both DCs and CD8+ T cells and potentiated the antitumor efficacy of anti-PD1 antibody.
Conclusions: Our findings showed that clotrimazole could trigger DC activation via the lactate-lysosome axis to promote antigen cross-presentation and could be used as a potential combination therapy approach to improving the therapeutic efficacy of anti-PD1 immunotherapy.
Keywords: dendritic cells; immunotherapy.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures



Similar articles
-
DNGR-1 limits Flt3L-mediated antitumor immunity by restraining tumor-infiltrating type I conventional dendritic cells.J Immunother Cancer. 2021 May;9(5):e002054. doi: 10.1136/jitc-2020-002054. J Immunother Cancer. 2021. PMID: 33980589 Free PMC article.
-
In situ delivery of iPSC-derived dendritic cells with local radiotherapy generates systemic antitumor immunity and potentiates PD-L1 blockade in preclinical poorly immunogenic tumor models.J Immunother Cancer. 2021 May;9(5):e002432. doi: 10.1136/jitc-2021-002432. J Immunother Cancer. 2021. PMID: 34049930 Free PMC article.
-
STING agonist-based treatment promotes vascular normalization and tertiary lymphoid structure formation in the therapeutic melanoma microenvironment.J Immunother Cancer. 2021 Feb;9(2):e001906. doi: 10.1136/jitc-2020-001906. J Immunother Cancer. 2021. PMID: 33526609 Free PMC article.
-
Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment.Front Immunol. 2018 Dec 20;9:3059. doi: 10.3389/fimmu.2018.03059. eCollection 2018. Front Immunol. 2018. PMID: 30619378 Free PMC article. Review.
-
Dendritic Cells, the T-cell-inflamed Tumor Microenvironment, and Immunotherapy Treatment Response.Clin Cancer Res. 2020 Aug 1;26(15):3901-3907. doi: 10.1158/1078-0432.CCR-19-1321. Epub 2020 Apr 24. Clin Cancer Res. 2020. PMID: 32332013 Free PMC article. Review.
Cited by
-
Fungi and cancer: unveiling the complex role of fungal infections in tumor biology and therapeutic resistance.Front Cell Infect Microbiol. 2025 Jun 10;15:1596688. doi: 10.3389/fcimb.2025.1596688. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40557321 Free PMC article. Review.
-
Targeting the Warburg Effect in Cancer: Where Do We Stand?Int J Mol Sci. 2024 Mar 8;25(6):3142. doi: 10.3390/ijms25063142. Int J Mol Sci. 2024. PMID: 38542116 Free PMC article. Review.
-
From metabolic byproduct to immune modulator: the role of lactate in tumor immune escape.Front Immunol. 2024 Nov 25;15:1492050. doi: 10.3389/fimmu.2024.1492050. eCollection 2024. Front Immunol. 2024. PMID: 39654883 Free PMC article. Review.
-
The significance of targeting lysosomes in cancer immunotherapy.Front Immunol. 2024 Feb 2;15:1308070. doi: 10.3389/fimmu.2024.1308070. eCollection 2024. Front Immunol. 2024. PMID: 38370407 Free PMC article. Review.
-
Pyrithione zinc alters mismatch repair to trigger tumor immunogenicity.Oncogene. 2025 Apr;44(14):983-995. doi: 10.1038/s41388-024-03272-1. Epub 2025 Jan 15. Oncogene. 2025. PMID: 39814851
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
