Sequential infection with H1N1 and SARS-CoV-2 aggravated COVID-19 pathogenesis in a mammalian model, and co-vaccination as an effective method of prevention of COVID-19 and influenza
- PMID: 34016949
- PMCID: PMC8134832
- DOI: 10.1038/s41392-021-00618-z
Sequential infection with H1N1 and SARS-CoV-2 aggravated COVID-19 pathogenesis in a mammalian model, and co-vaccination as an effective method of prevention of COVID-19 and influenza
Abstract
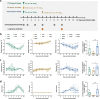
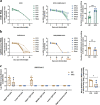
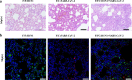
Influenza A virus may circulate simultaneously with the SARS-CoV-2 virus, leading to more serious respiratory diseases during this winter. However, the influence of these viruses on disease outcome when both influenza A and SARS-CoV-2 are present in the host remains unclear. Using a mammalian model, sequential infection was performed in ferrets and in K18-hACE2 mice, with SARS-CoV-2 infection following H1N1. We found that co-infection with H1N1 and SARS-CoV-2 extended the duration of clinical manifestation of COVID-19, and enhanced pulmonary damage, but reduced viral shedding of throat swabs and viral loads in the lungs of ferrets. Moreover, mortality was increased in sequentially infected mice compared with single-infection mice. Compared with single-vaccine inoculation, co-inoculation of PiCoVacc (a SARS-CoV-2 vaccine) and the flu vaccine showed no significant differences in neutralizing antibody titers or virus-specific immune responses. Combined immunization effectively protected K18-hACE2 mice against both H1N1 and SARS-CoV-2 infection. Our findings indicated the development of systematic models of co-infection of H1N1 and SARS-CoV-2, which together notably enhanced pneumonia in ferrets and mice, as well as demonstrated that simultaneous vaccination against H1N1 and SARS-CoV-2 may be an effective prevention strategy for the coming winter.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

