NRG Oncology/NSABP B-47 menstrual history study: impact of adjuvant chemotherapy with and without trastuzumab
- PMID: 34016989
- PMCID: PMC8137688
- DOI: 10.1038/s41523-021-00264-2
NRG Oncology/NSABP B-47 menstrual history study: impact of adjuvant chemotherapy with and without trastuzumab
Abstract
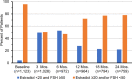
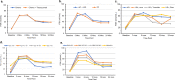
The NRG Oncology/NSABP B-47 menstrual history (MH) study examined trastuzumab effects on menstrual status and associated circulating reproductive hormones. MH was evaluated by questions related to hysterectomy, oophorectomy, and reported menstrual changes. Pre/perimenopausal women were assessed at entry, 3, 6, 12, 18, 24, 30, and 36 months. Consenting women had estradiol and FSH measurement at entry, 3, 6, 12, 18, and 24 months. Logistic regression determined predictors of amenorrhea and hormone levels at 12, 24, and 36 months. Between 2/8/2011 and 2/10/2015, 3270 women with node-positive/high-risk node-negative HER2-low breast cancer were enrolled. There were 1,458 women enrolled in the MH study; 1231 consented to baseline blood samples. Trastuzumab did not contribute to a higher amenorrhea rate. Amenorrhea predictors were consistent with earlier studies; however, to our knowledge, this is the largest prospective study to include serial reproductive hormone measurements to 24 months and clinical amenorrhea reports to 36 months. These data can help to counsel patients regarding premature menopause risk.
Conflict of interest statement
J.F.B.: Jewish General Hospital/Segal Cancer Centre receives funding for research from Roche – JF Boileau, PI; Consultant for Roche; participated on Advisory Boards for Roche. J.P.C.: Research funding (to institution): Eisai, Puma Biotechnology, Roche, Boehringer Ingelheim; Employment: OncoMark, Ltd.; Honoraria: Eisai, Puma Biotechnology; MSD Oncology, Pfizer, G1 Therapeutics; Novartis; Speaker’s Bureau: Boehringer Ingelheim, Genomic Health, Roche, Pfizer; Shares: OncoMark Ltd; Travel and accommodation expenses: Pfizer, MSD, Abbvie, Astrazeneca, Novartis. P.A.G.: member of the Breast Cancer Research Foundation scientific advisory board. E.P.M.: Consultant: Genentech/Roche, Exact Sciences, Daiichi Sankyo, Biotheranostics, Puma Biotechnology, Merk; Speaker’s Bureau: Genentech/Roche, Exact Sciences. P.R.: Travel and accommodations, Genentech/Roche, AstraZeneca, and Lilly. N.W.: Research Funding to institution: AstraZeneca/MedImmune (Inst), NSABP Foundation (Inst). All remaining authors declare no competing interests.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

