CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer
- PMID: 34017137
- PMCID: PMC8291091
- DOI: 10.1038/s41591-021-01386-7
CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer
Abstract
Patients with cancer have high mortality from coronavirus disease 2019 (COVID-19), and the immune parameters that dictate clinical outcomes remain unknown. In a cohort of 100 patients with cancer who were hospitalized for COVID-19, patients with hematologic cancer had higher mortality relative to patients with solid cancer. In two additional cohorts, flow cytometric and serologic analyses demonstrated that patients with solid cancer and patients without cancer had a similar immune phenotype during acute COVID-19, whereas patients with hematologic cancer had impairment of B cells and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific antibody responses. Despite the impaired humoral immunity and high mortality in patients with hematologic cancer who also have COVID-19, those with a greater number of CD8 T cells had improved survival, including those treated with anti-CD20 therapy. Furthermore, 77% of patients with hematologic cancer had detectable SARS-CoV-2-specific T cell responses. Thus, CD8 T cells might influence recovery from COVID-19 when humoral immunity is deficient. These observations suggest that CD8 T cell responses to vaccination might provide protection in patients with hematologic cancer even in the setting of limited humoral responses.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures










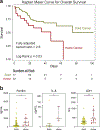


Update of
-
CD8 T cells compensate for impaired humoral immunity in COVID-19 patients with hematologic cancer.Res Sq [Preprint]. 2021 Feb 2:rs.3.rs-162289. doi: 10.21203/rs.3.rs-162289/v1. Res Sq. 2021. Update in: Nat Med. 2021 Jul;27(7):1280-1289. doi: 10.1038/s41591-021-01386-7. PMID: 33564756 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA009512/CA/NCI NIH HHS/United States
- R01 HL137006/HL/NHLBI NIH HHS/United States
- U19 AI117950/AI/NIAID NIH HHS/United States
- T32 CA009140/CA/NCI NIH HHS/United States
- R01 AI155577/AI/NIAID NIH HHS/United States
- K08 CA230157/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U19 AI082630/AI/NIAID NIH HHS/United States
- P01 AI112521/AI/NIAID NIH HHS/United States
- T32 CA009679/CA/NCI NIH HHS/United States
- R01 AI123539/AI/NIAID NIH HHS/United States
- K08 AI136660/AI/NIAID NIH HHS/United States
- R01 HL137915/HL/NHLBI NIH HHS/United States
- T32 HL007586/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

