Pharmacokinetics and Pharmacodynamics of Nemonoxacin in a Neutropenic Murine Lung Infection Model Against Streptococcus Pneumoniae
- PMID: 34017256
- PMCID: PMC8129567
- DOI: 10.3389/fphar.2021.658558
Pharmacokinetics and Pharmacodynamics of Nemonoxacin in a Neutropenic Murine Lung Infection Model Against Streptococcus Pneumoniae
Abstract
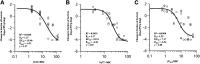
Nemonoxacin, a novel nonfluorinated quinolone for the treatment of community-acquired pneumonia. We reported the pharmacokinetic/pharmacodynamic (PK/PD) targets and PK/PD breakpoints of nemonoxacin against Streptococcus pneumoniae using a neutropenic murine lung infection model. Single-dose PK analysis after subcutaneous administration of nemonoxacin at doses from 2.5 to 80 mg/kg showed maximum plasma concentration (Cmax) 0.56-7.32 mg/L, area under the concentration-time curve from 0 to 24 h (AUC0-24) 0.67-26.10 mg·h/L, and elimination half-life (T1/2) 0.8-1.4 h. The epithelial lining fluid (ELF) penetration ratio of total drug was 1.40. Dose fractionation (1.25-80 mg/kg/day, every 24, 12, 8, and 6 h) and dose escalation studies (1.25-160 mg/kg, every 24 h) were conducted. The sigmoid Emax Hill equation was used to describe the dose-response data. The free-drug plasma fAUC0-24/MIC ratio was considered the PK/PD index most closely associated with efficacy (R2 0.9268). Median fAUC0-24/MIC associated with static, 1-log10 and 2-log10 CFU reduction from baseline were 8.6, 23.2 and 44.4, respectively. Monte Carlo simulation showed 500 mg qd and 750 mg qd oral doses of nemonoxacin were able to achieve 90% probability of target attainment (PTA) against bacteria with MIC of 0.5 mg/L and 1 mg/L. We recommended susceptibility (S) ≤ 0.5 mg/L, intermediate (I) = 1 mg/L and resistant (R) ≥ 2 mg/L as the PK/PD breakpoints for nemonoxacin against S. pneumoniae.
Keywords: breakpoint; nemonoxacin; neutropenic murine lung infection model; pharmacokinetics/pharmacodynamics; streptococcus pneumoniae.
Copyright © 2021 Li, Chen, Xu, Li, Fan, Liu, Bian, Wu, Zhao, Feng, Guo and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Ambrose P. G., Grasela D. M., Grasela T. H., Passarell J., Mayer H. B., Pierce P. F. (2001). Pharmacodynamics of Fluoroquinolones against Streptococcus Pneumoniae in Patients with Community-Acquired Respiratory Tract Infections. Antimicrob. Agents Chemother. 45, 2793–2797. 10.1128/AAC.45.10.2793-2797.200110.1128/aac.45.10.2793-2797.2001 - DOI - PMC - PubMed
-
- Berkhout J., Melchers M. J., Van Mil A. C., Seyedmousavi S., Lagarde C. M., Nichols W. W., et al. (2015). Pharmacokinetics and Penetration of Ceftazidime and Avibactam into Epithelial Lining Fluid in Thigh- and Lung-Infected Mice. Antimicrob. Agents Chemother. 59, 2299–2304. 10.1128/aac.04627-14 - DOI - PMC - PubMed
-
- Bulik C. C., Okusanya Ó. O., Lakota E. A., Forrest A., Bhavnani S. M., Hoover J. L., et al. (2017). Pharmacokinetic-Pharmacodynamic Evaluation of Gepotidacin against Gram-Positive Organisms Using Data from Murine Infection Models. Antimicrob. Agents Chemother. 61, e00115-16. 10.1128/AAC - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

