Continuous MYD88 Activation Is Associated With Expansion and Then Transformation of IgM Differentiating Plasma Cells
- PMID: 34017329
- PMCID: PMC8129569
- DOI: 10.3389/fimmu.2021.641692
Continuous MYD88 Activation Is Associated With Expansion and Then Transformation of IgM Differentiating Plasma Cells
Abstract
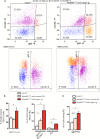
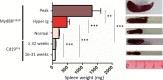
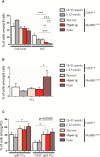
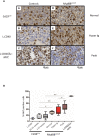
Activating mutations of MYD88 (MYD88L265P being the far most frequent) are found in most cases of Waldenström macroglobulinemia (WM) as well as in various aggressive B-cell lymphoma entities with features of plasma cell (PC) differentiation, such as activated B-cell type diffuse large B-cell lymphoma (DLBCL). To understand how MYD88 activation exerts its transformation potential, we developed a new mouse model in which the MYD88L252P protein, the murine ortholog of human MYD88L265P, is continuously expressed in CD19 positive B-cells together with the Yellow Fluorescent Protein (Myd88L252P mice). In bone marrow, IgM B and plasma cells were expanded with a CD138 expression continuum from IgMhigh CD138low to IgMlow CD138high cells and the progressive loss of the B220 marker. Serum protein electrophoresis (SPE) longitudinal analysis of 40 Myd88L252P mice (16 to 56 weeks old) demonstrated that ageing was first associated with serum polyclonal hyper gammaglobulinemia (hyper Ig) and followed by a monoclonal immunoglobulin (Ig) peak related to a progressive increase in IgM serum levels. All Myd88L252P mice exhibited spleen enlargement which was directly correlated with the SPE profile and was maximal for monoclonal Ig peaks. Myd88L252P mice exhibited very early increased IgM PC differentiation. Most likely due to an early increase in the Ki67 proliferation index, IgM lymphoplasmacytic (LP) and plasma cells continuously expanded with age being first associated with hyper Ig and then with monoclonal Ig peak. This peak was consistently associated with a spleen LP-like B-cell lymphoma. Clonal expression of both membrane and secreted µ chain isoforms was demonstrated at the mRNA level by high throughput sequencing. The Myd88L252P tumor transcriptomic signature identified both proliferation and canonical NF-κB p65/RelA activation. Comparison with MYD88L265P WM showed that Myd88L252P tumors also shared the typical lymphoplasmacytic transcriptomic signature of WM bone marrow purified tumor B-cells. Altogether these results demonstrate for the first time that continuous MYD88 activation is specifically associated with clonal transformation of differentiating IgM B-cells. Since MYD88L252P targets the IgM PC differentiation continuum, it provides an interesting preclinical model for development of new therapeutic approaches to both WM and aggressive MYD88 associated DLBCLs.
Keywords: B-cell lymphoma; IgM secretion; MYD88 L265P mutation; lymphoplasmacytic lymphoma/Waldenstrom’s macroglobulinemia; monoclonal Ig peak; plasma cell.
Copyright © 2021 Ouk, Roland, Gachard, Poulain, Oblet, Rizzo, Saintamand, Lemasson, Carrion, Thomas, Balabanian, Espéli, Parrens, Soubeyran, Boulin, Faumont, Feuillard and Vincent-Fabert.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

