Rational Design of Peptide-Based Inhibitors Disrupting Protein-Protein Interactions
- PMID: 34017824
- PMCID: PMC8128998
- DOI: 10.3389/fchem.2021.682675
Rational Design of Peptide-Based Inhibitors Disrupting Protein-Protein Interactions
Abstract
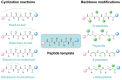
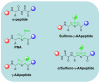
Protein-protein interactions (PPIs) are well-established as a class of promising drug targets for their implications in a wide range of biological processes. However, drug development toward PPIs is inevitably hampered by their flat and wide interfaces, which generally lack suitable pockets for ligand binding, rendering most PPI systems "undruggable." Here, we summarized drug design strategies for developing peptide-based PPI inhibitors. Importantly, several quintessential examples toward well-established PPI targets such as Bcl-2 family members, p53-MDM2, as well as APC-Asef are presented to illustrate the detailed schemes for peptide-based PPI inhibitor development and optimizations. This review supplies a comprehensive overview of recent progresses in drug discovery targeting PPIs through peptides or peptidomimetics, and will shed light on future therapeutic agent development toward the historically "intractable" PPI systems.
Keywords: drug discovery; peptide; peptidomimetics; protein-protein interaction; undruggable.
Copyright © 2021 Wang, Ni, Liu and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Beekman A. M., O'Connell M. A., Howell L. A. (2017). Peptide-directed binding for the discovery of modulators of alpha-helix-mediated protein-protein interactions: proof-of-concept studies with the apoptosis regulator Mcl-1. Angew. Chem. Int. Ed. Engl. 56, 10446–10450. 10.1002/anie.201705008 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

