Role of ex vivo Expanded Mesenchymal Stromal Cells in Determining Hematopoietic Stem Cell Transplantation Outcome
- PMID: 34017834
- PMCID: PMC8129582
- DOI: 10.3389/fcell.2021.663316
Role of ex vivo Expanded Mesenchymal Stromal Cells in Determining Hematopoietic Stem Cell Transplantation Outcome
Abstract
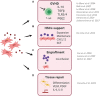
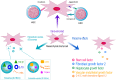
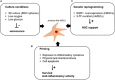
Overall, the human organism requires the production of ∼1 trillion new blood cells per day. Such goal is achieved via hematopoiesis occurring within the bone marrow (BM) under the tight regulation of hematopoietic stem and progenitor cell (HSPC) homeostasis made by the BM microenvironment. The BM niche is defined by the close interactions of HSPCs and non-hematopoietic cells of different origin, which control the maintenance of HSPCs and orchestrate hematopoiesis in response to the body's requirements. The activity of the BM niche is regulated by specific signaling pathways in physiological conditions and in case of stress, including the one induced by the HSPC transplantation (HSCT) procedures. HSCT is the curative option for several hematological and non-hematological diseases, despite being associated with early and late complications, mainly due to a low level of HSPC engraftment, impaired hematopoietic recovery, immune-mediated graft rejection, and graft-versus-host disease (GvHD) in case of allogenic transplant. Mesenchymal stromal cells (MSCs) are key elements of the BM niche, regulating HSPC homeostasis by direct contact and secreting several paracrine factors. In this review, we will explore the several mechanisms through which MSCs impact on the supportive activity of the BM niche and regulate HSPC homeostasis. We will further discuss how the growing understanding of such mechanisms have impacted, under a clinical point of view, on the transplantation field. In more recent years, these results have instructed the design of clinical trials to ameliorate the outcome of HSCT, especially in the allogenic setting, and when low doses of HSPCs were available for transplantation.
Keywords: bone marrow niche; hematopoietic (stem) cell transplantation; hematopoietic stem and progenitor cell; immunoregulation; mesenchymal stromal cells.
Copyright © 2021 Crippa, Santi, Berti, De Ponti and Bernardo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Bone Marrow-Derived Mesenchymal Stromal Cells: A Novel Target to Optimize Hematopoietic Stem Cell Transplantation Protocols in Hematological Malignancies and Rare Genetic Disorders.J Clin Med. 2019 Dec 18;9(1):2. doi: 10.3390/jcm9010002. J Clin Med. 2019. PMID: 31861268 Free PMC article. Review.
-
Mesenchymal Stromal Cells: Role in the BM Niche and in the Support of Hematopoietic Stem Cell Transplantation.Hemasphere. 2018 Nov 16;2(6):e151. doi: 10.1097/HS9.0000000000000151. eCollection 2018 Dec. Hemasphere. 2018. PMID: 31723790 Free PMC article. Review.
-
TGFBI Expressed by Bone Marrow Niche Cells and Hematopoietic Stem and Progenitor Cells Regulates Hematopoiesis.Stem Cells Dev. 2018 Nov 1;27(21):1494-1506. doi: 10.1089/scd.2018.0124. Epub 2018 Sep 6. Stem Cells Dev. 2018. PMID: 30084753 Free PMC article.
-
Hematopoietic Niche - Exploring Biomimetic Cues to Improve the Functionality of Hematopoietic Stem/Progenitor Cells.Biotechnol J. 2018 Feb;13(2). doi: 10.1002/biot.201700088. Epub 2017 Dec 28. Biotechnol J. 2018. PMID: 29178199 Review.
-
Neurological Regulation of the Bone Marrow Niche.Adv Exp Med Biol. 2020;1212:127-153. doi: 10.1007/5584_2019_398. Adv Exp Med Biol. 2020. PMID: 31342461 Review.
Cited by
-
Telomeres and Telomerase in the Control of Stem Cells.Biomedicines. 2022 Sep 20;10(10):2335. doi: 10.3390/biomedicines10102335. Biomedicines. 2022. PMID: 36289597 Free PMC article. Review.
-
Mesenchymal stem cells and their derived exosomes in multiple sclerosis disease: from paper to practice.Mol Cell Biochem. 2024 Jul;479(7):1643-1671. doi: 10.1007/s11010-024-05051-8. Epub 2024 Jul 8. Mol Cell Biochem. 2024. PMID: 38977625 Review.
-
Mesenchymal Stromal Cells for the Treatment of Graft Versus Host Disease.Front Immunol. 2021 Oct 26;12:761616. doi: 10.3389/fimmu.2021.761616. eCollection 2021. Front Immunol. 2021. PMID: 34764962 Free PMC article. Review.
-
Transcriptomic analysis of BM-MSCs identified EGR1 as a transcription factor to fully exploit their therapeutic potential.Biochim Biophys Acta Mol Cell Res. 2024 Dec;1871(8):119818. doi: 10.1016/j.bbamcr.2024.119818. Epub 2024 Aug 19. Biochim Biophys Acta Mol Cell Res. 2024. PMID: 39168411 Free PMC article.
-
Mesenchymal Stromal Cells from Perinatal Tissues as an Alternative for Ex Vivo Expansion of Hematopoietic Progenitor and Stem Cells from Umbilical Cord Blood.Int J Mol Sci. 2023 Oct 24;24(21):15544. doi: 10.3390/ijms242115544. Int J Mol Sci. 2023. PMID: 37958529 Free PMC article.
References
-
- Abbuehl J. P., Tatarova Z., Held W., Huelsken J. (2017). Long-Term engraftment of primary bone marrow stromal cells repairs niche damage and improves hematopoietic stem cell transplantation. Cell Stem Cell 21 241–255.e6. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

