The Long-Term Impact of Levodopa/Carbidopa Intestinal Gel on 'Off'-time in Patients with Advanced Parkinson's Disease: A Systematic Review
- PMID: 34018146
- PMCID: PMC8189983
- DOI: 10.1007/s12325-021-01747-1
The Long-Term Impact of Levodopa/Carbidopa Intestinal Gel on 'Off'-time in Patients with Advanced Parkinson's Disease: A Systematic Review
Abstract
Introduction: Levodopa/carbidopa intestinal gel (LCIG; carbidopa/levodopa enteral suspension) has been widely used and studied for the treatment of motor fluctuations in levodopa-responsive patients with advanced Parkinson's disease (PD) when other treatments have not given satisfactory results. Reduction in 'off'-time is a common primary endpoint in studies of LCIG, and it is important to assess the durability of this response. This systematic literature review was conducted to qualitatively summarise the data on the long-term effects of LCIG therapy on 'off'-time.
Methods: Studies were identified by searching PubMed, EMBASE and Ovid on 30 September 2019. Studies were included if they reported on patients with PD, had a sample size of ≥ 10, LCIG was an active intervention and 'off'-time was reported for ≥ 12 months after initiation of LCIG treatment. Randomised clinical trials, retrospective and prospective observational studies, and other interventional studies were included for selection. Data were collected on: 'off'-time (at pre-specified time periods and the end of follow-up), study characteristics, Unified Parkinson's Disease Rating Scale (UPDRS) II, III and IV total scores, dyskinesia duration, quality of life scores, non-motor symptoms and safety outcomes.
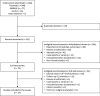
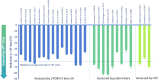
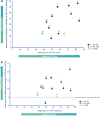
Results: Twenty-seven studies were included in this review. The improvement in 'off'-time observed shortly after initiating LCIG was maintained and was statistically significant at the end of follow-up in 24 of 27 studies. 'Off'-time was reduced from baseline to end of follow-up by 38-84% and was accompanied by a clinically meaningful improvement in quality of life. Stratified analysis of 'off'-time demonstrated mean relative reductions of 47-82% at 3-6 months and up to 83% reduction at 3-5 years of follow-up. Most studies reported significant improvements in activities of daily living and motor complications. Most frequent adverse events were related to the procedure or the device.
Conclusion: In one of the largest qualitative syntheses of published LCIG studies, LCIG treatment was observed to provide a durable effect in reducing 'off'-time.
Infographic: Video Abstract.
Keywords: Advanced Parkinson’s disease; LCIG; Long-term; ‘Off’-time.
Plain language summary
By synthesising publications from scientific journals, this article shows that levodopa/carbidopa intestinal gel (LCIG; also known as carbidopa/levodopa enteral suspension or the tradenames Duodopa® and Duopa®) may have benefits for patients with advanced Parkinson’s disease that last for 12 months or more. Pills taken by mouth for Parkinson’s disease often do not work as well after a few years. This means the symptoms of Parkinson’s disease, such as shaking or slow movements, etc., re-emerge despite medication (known as ‘off’-time). To reduce the amount of ‘off’-time, people with advancing Parkinson’s disease may switch from pills to other types of treatments, for example, those that use devices to deliver the drug into the body, such as LCIG. LCIG has been available for many years and is known to help patients by reducing ‘off’-time. Despite this, less is known about how long the benefits of LCIG last. By summarising all information available on the long-term use of LCIG, this report shows that when patients have been taking LCIG for at least 12 months, they have 2–4 h less ‘off’-time each day than they did before starting the LCIG treatment. This effect is maintained for 3–5 years after starting LCIG treatment. There were no unexpected side effects with long-term use of LCIG. The time not spent in ‘off’ may allow people with advanced Parkinson’s to increase their independence in daily activities.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

