The Longitudinal Early-onset Alzheimer's Disease Study (LEADS): Framework and methodology
- PMID: 34018654
- PMCID: PMC8939858
- DOI: 10.1002/alz.12350
The Longitudinal Early-onset Alzheimer's Disease Study (LEADS): Framework and methodology
Abstract
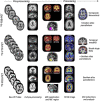
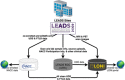
Patients with early-onset Alzheimer's disease (EOAD) are commonly excluded from large-scale observational and therapeutic studies due to their young age, atypical presentation, or absence of pathogenic mutations. The goals of the Longitudinal EOAD Study (LEADS) are to (1) define the clinical, imaging, and fluid biomarker characteristics of EOAD; (2) develop sensitive cognitive and biomarker measures for future clinical and research use; and (3) establish a trial-ready network. LEADS will follow 400 amyloid beta (Aβ)-positive EOAD, 200 Aβ-negative EOnonAD that meet National Institute on Aging-Alzheimer's Association (NIA-AA) criteria for mild cognitive impairment (MCI) or AD dementia, and 100 age-matched controls. Participants will undergo clinical and cognitive assessments, magnetic resonance imaging (MRI), [18 F]Florbetaben and [18 F]Flortaucipir positron emission tomography (PET), lumbar puncture, and blood draw for DNA, RNA, plasma, serum and peripheral blood mononuclear cells, and post-mortem assessment. To develop more effective AD treatments, scientists need to understand the genetic, biological, and clinical processes involved in EOAD. LEADS will develop a public resource that will enable future planning and implementation of EOAD clinical trials.
Keywords: Alzheimer's disease; EOAD; LEADS; YOAD; early-onset; young onset.
© 2021 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Dr. Apostolova served as a paid consultant for Biogen and Two Labs, serves on a DSMB for IQVIA, and receives research support from the NIH, the Alzheimer's Association, Roche, Life Molecular Imaging, and Eli Lilly. Dr. Aisen reports grants from NIA, FNIH, the Alzheimer's Association, Janssen, Lilly, and Eisai, and personal fees from Merck, Roche, Biogen, Lundbeck, ImmunoBrain Checkpoint, and Samus. Dr. Eloyan has nothing to disclose. Dr. Fagan has received research support from Biogen, Fujirebio, and Roche Diagnostics. She is a member of the scientific advisory boards for Roche Diagnostics, Genentech, and AbbVie and also consults for Araclon/Griffols, DiademRes, and Otsuka Pharmaceuticals. Dr. Fargo has nothing to disclose. Dr. Foroud has nothing to disclose. Dr. Gatsonis has nothing to disclose. Dr. Grinberg has nothing to disclose. Dr. Jack serves on an independent data monitoring board for Roche and has consulted for and served as a speaker for Eisai, but he receives no personal compensation from any commercial entity. He receives research support from the NIH and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Clinic. Dr. Kramer has nothing to disclose. Dr. Koeppe has nothing to disclose. Dr. Kukull is supported by the NIA. Dr. Murray served as a paid consultant for AVID Radiopharmaceuticals and receives research support from the NIH, the Alzheimer's Association, and the state of Florida. Dr. Nudelman has nothing to disclose. Mrs. Rumbaugh has nothing to disclose. Dr. Toga has nothing to disclose. Dr. Vemuri has nothing to disclose. Mrs. Trullinger has nothing to disclose. Dr. Iaccarino has nothing to disclose. Dr. Day is supported by the NIA; he serves as a topic editor on dementia for DynaMed Plus (EBSCO Industries, Inc), is the clinical director for the Anti‐NMDA Receptor Encephalitis Foundation and holds stocks in ANI Pharmaceuticals. Dr. Graff‐Radford receives research support from Lilly, AbbVie, and Biogen. Dr. Honig has nothing to disclose. Dr. Jones has nothing to disclose. Dr. Masdeu served on the speaker's bureau and consulted for Biogen. He received research support from the NIH, Avanir, Abbvie, Biogen, Eli Lilly, Esai, and Novartis. Dr. Mendez receives grant support from NIA and has received support from Biogen. Dr. Musiek received research funding from Eisai Pharmaceuticals Inc. Dr. Onyike has nothing to disclose. Dr. Rogalski has nothing to disclose. Dr. Salloway received consultation fees and research support from Biogen, Eisai, Lilly, Genentech, and Roche. Dr. Wolk received grants from Eli Lilly/Avid Radiopharmaceuticals, Merck, and Biogen, and consulted for Merck, Janssen, and GE Healthcare. Dr. Wingo has nothing to disclose. Dr. Carrillo has nothing to disclose. Dr. Dickerson receives research support from the NIH and the Alzheimer's Drug Discovery Foundation. He consulted for Arkuda, Axovant, Lilly, Biogen, Merck, Novartis, and Wave LifeSciences. He is editor for
Figures
References
-
- Alzheimer Association . 2019 Alzheimer's disease facts and figures. Alzheimers Dement 2019;15(3):321‐387.
-
- Alzheimer Association . 2020 Alzheimer's disease facts and figures. Alzheimers Dement 2020;16(3):391‐460.
-
- van Vliet D, de Vugt ME, Bakker C, et al. Impact of early onset dementia on caregivers: a review. Int J Geriatr Psychiatry. 2010;25(11):1091‐1100. - PubMed
-
- Werner P, Stein‐Shvachman I, Korczyn AD. Early onset dementia: clinical and social aspects. Int Psychogeriatr. 2009;21(4):631‐636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG013854/AG/NIA NIH HHS/United States
- P30 AG066507/AG/NIA NIH HHS/United States
- R56 AG057195/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- P30 AG066462/AG/NIA NIH HHS/United States
- P30 AG066530/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- P30 AG072979/AG/NIA NIH HHS/United States
- P41 EB015922/EB/NIBIB NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- U01 AG057195/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- P30 AG066511/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- U24 AG072122/AG/NIA NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P30 AG072977/AG/NIA NIH HHS/United States
- P30 AG062677/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical