Tocilizumab in COVID-19: a meta-analysis, trial sequential analysis, and meta-regression of randomized-controlled trials
- PMID: 34019122
- PMCID: PMC8139226
- DOI: 10.1007/s00134-021-06416-z
Tocilizumab in COVID-19: a meta-analysis, trial sequential analysis, and meta-regression of randomized-controlled trials
Abstract
Purpose: Interleukin-6 (IL-6) levels discriminate between patients with mild and severe COVID-19, making IL-6 inhibition an attractive therapeutic strategy. We conducted a systematic review, meta-analysis, trial sequential analysis (TSA), and meta-regression of randomized-controlled trials to ascertain the benefit of IL-6 blockade with tocilizumab for COVID-19.
Methods: We included randomized-controlled trials (RCTs) allocating patients with COVID-19 to tocilizumab. Our control group included standard care or placebo. Trials co-administering other pharmacological interventions for COVID-19 were not excluded. Primary outcome was 28-30 day mortality. Secondary outcomes included progression-to-severe disease defined as need for mechanical ventilation, intensive-care unit (ICU) admission, or a composite.
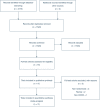
Results: We identified 10 RCTs using tocilizumab, 9 of which reported primary outcome data (mortality), recruiting 6493 patients with 3358 (52.2%) allocated to tocilizumab. Tocilizumab may be associated with an improvement in mortality (24.4% vs. 29.0%; OR 0.87 [0.74-1.01]; p = 0.07; I2 = 10%; TSA adjusted CI 0.66-1.14). Meta-regression suggested a relationship between treatment effect and mortality risk, with benefit at higher levels of risk (logOR vs %risk beta = -0.018 [-0.037 to -0.002]; p = 0.07). Tocilizumab did reduce the need for mechanical ventilation and was associated with a benefit in the composite secondary outcome but did not reduce ICU admission.
Conclusions: For hospitalized COVID-19 patients, there is some evidence that tocilizumab use may be associated with a short-term mortality benefit, but further high-quality data are required. Its benefits may also lie in reducing the need for mechanical ventilation.
Keywords: COVID-19; Immunologic factors; Interleukin-6; Meta-analysis.
Conflict of interest statement
MS reports grants and advisory board fees from NewB, grants from the Defence Science and Technology Laboratory, Critical Pressure, Apollo Therapeutics, advisory board and speaker fees (paid to his institution) from Amormed, Biotest, GE, Baxter, Roche, and Bayer, and honorarium for chairing a data monitoring and safety committee from Shionogi.
Figures


Comment in
-
"L'histoire se répète", one size does not fit all.Intensive Care Med. 2021 Oct;47(10):1169-1170. doi: 10.1007/s00134-021-06483-2. Epub 2021 Jul 20. Intensive Care Med. 2021. PMID: 34286360 Free PMC article. No abstract available.
-
"L'histoire se répète", one size does not fit all. Author's reply.Intensive Care Med. 2021 Oct;47(10):1171-1172. doi: 10.1007/s00134-021-06497-w. Epub 2021 Aug 6. Intensive Care Med. 2021. PMID: 34363094 Free PMC article. No abstract available.
References
-
- Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

