Activation of Neuropeptide Y2 Receptor Can Inhibit Global Cerebral Ischemia-Induced Brain Injury
- PMID: 34019239
- PMCID: PMC8606017
- DOI: 10.1007/s12017-021-08665-z
Activation of Neuropeptide Y2 Receptor Can Inhibit Global Cerebral Ischemia-Induced Brain Injury
Abstract
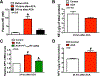
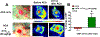
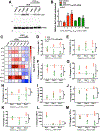
Cardiopulmonary arrest (CA) can greatly impact a patient's life, causing long-term disability and death. Although multi-faceted treatment strategies against CA have improved survival rates, the prognosis of CA remains poor. We previously reported asphyxial cardiac arrest (ACA) can cause excessive activation of the sympathetic nervous system (SNS) in the brain, which contributes to cerebral blood flow (CBF) derangements such as hypoperfusion and, consequently, neurological deficits. Here, we report excessive activation of the SNS can cause enhanced neuropeptide Y levels. In fact, mRNA and protein levels of neuropeptide Y (NPY, a 36-amino acid neuropeptide) in the hippocampus were elevated after ACA-induced SNS activation, resulting in a reduced blood supply to the brain. Post-treatment with peptide YY3-36 (PYY3-36), a pre-synaptic NPY2 receptor agonist, after ACA inhibited NPY release and restored brain circulation. Moreover, PYY3-36 decreased neuroinflammatory cytokines, alleviated mitochondrial dysfunction, and improved neuronal survival and neurological outcomes. Overall, NPY is detrimental during/after ACA, but attenuation of NPY release via PYY3-36 affords neuroprotection. The consequences of PYY3-36 inhibit ACA-induced 1) hypoperfusion, 2) neuroinflammation, 3) mitochondrial dysfunction, 4) neuronal cell death, and 5) neurological deficits. The present study provides novel insights to further our understanding of NPY's role in ischemic brain injury.
Keywords: Cerebral blood flow; Cerebral ischemia; Neurological deficits; Neuronal cell death; Neuropeptide Y.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Conflicts of interest/Competing interests
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures




References
-
- (2011). In th (Ed.), Guide for the Care and Use of Laboratory Animals (The National Academies Collection: Reports funded by National Institutes of Health; ). Washington (DC).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

