Real-life clinical sensitivity of SARS-CoV-2 RT-PCR test in symptomatic patients
- PMID: 34019562
- PMCID: PMC8139477
- DOI: 10.1371/journal.pone.0251661
Real-life clinical sensitivity of SARS-CoV-2 RT-PCR test in symptomatic patients
Abstract
Background: Understanding the false negative rates of SARS-CoV-2 RT-PCR testing is pivotal for the management of the COVID-19 pandemic and it has implications for patient management. Our aim was to determine the real-life clinical sensitivity of SARS-CoV-2 RT-PCR.
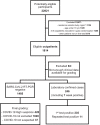
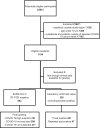
Methods: This population-based retrospective study was conducted in March-April 2020 in the Helsinki Capital Region, Finland. Adults who were clinically suspected of SARS-CoV-2 infection and underwent SARS-CoV-2 RT-PCR testing, with sufficient data in their medical records for grading of clinical suspicion were eligible. In addition to examining the first RT-PCR test of repeat-tested individuals, we also used high clinical suspicion for COVID-19 as the reference standard for calculating the sensitivity of SARS-CoV-2 RT-PCR.
Results: All 1,194 inpatients (mean [SD] age, 63.2 [18.3] years; 45.2% women) admitted to COVID-19 cohort wards during the study period were included. The outpatient cohort of 1,814 individuals (mean [SD] age, 45.4 [17.2] years; 69.1% women) was sampled from epidemiological line lists by systematic quasi-random sampling. The sensitivity (95% CI) for laboratory confirmed cases (repeat-tested patients) was 85.7% (81.5-89.1%) inpatients; 95.5% (92.2-97.5%) outpatients, 89.9% (88.2-92.1%) all. When also patients that were graded as high suspicion but never tested positive were included in the denominator, the sensitivity (95% CI) was: 67.5% (62.9-71.9%) inpatients; 34.9% (31.4-38.5%) outpatients; 47.3% (44.4-50.3%) all.
Conclusions: The clinical sensitivity of SARS-CoV-2 RT-PCR testing was only moderate at best. The relatively high false negative rates of SARS-CoV-2 RT-PCR testing need to be accounted for in clinical decision making, epidemiological interpretations, and when using RT-PCR as a reference for other tests.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: Dr. Kortela reports non-financial support from MSD, outside the submitted work. Prof. Järvinen reports lecture honoraria from Astellas, OrionPharma, Pfizer, MSD, Sanofi and UnimedicPharma and consultation fee from CSL Behring outside the submitted manuscript. Dr. Kekäläinen reports a lecture honorarium from MSD. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- WHO Overview of public health and social measures in the context of COVID-19. Available from https://www.who.int/publications/i/item/overview-of-public-health-and-so....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

