Turnover rate of coenzyme A in mouse brain and liver
- PMID: 34019583
- PMCID: PMC8139499
- DOI: 10.1371/journal.pone.0251981
Turnover rate of coenzyme A in mouse brain and liver
Abstract
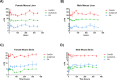
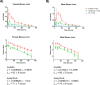
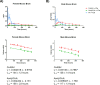
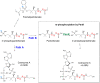
Coenzyme A (CoA) is a fundamental cofactor involved in a number of important biochemical reactions in the cell. Altered CoA metabolism results in severe conditions such as pantothenate kinase-associated neurodegeneration (PKAN) in which a reduction of the activity of pantothenate kinase isoform 2 (PANK2) present in CoA biosynthesis in the brain consequently lowers the level of CoA in this organ. In order to develop a new drug aimed at restoring the sufficient amount of CoA in the brain of PKAN patients, we looked at its turnover. We report here the results of two experiments that enabled us to measure the half-life of pantothenic acid, free CoA (CoASH) and acetylCoA in the brains and livers of male and female C57BL/6N mice, and total CoA in the brains of male mice. We administered (intrastriatally or orally) a single dose of a [13C3-15N-18O]-labelled coenzyme A precursor (fosmetpantotenate or [13C3-15N]-pantothenic acid) to the mice and measured, by liquid chromatography-mass spectrometry, unlabelled- and labelled-coenzyme A species appearance and disappearance over time. We found that the turnover of all metabolites was faster in the liver than in the brain in both genders with no evident gender difference observed. In the oral study, the CoASH half-life was: 69 ± 5 h (male) and 82 ± 6 h (female) in the liver; 136 ± 14 h (male) and 144 ± 12 h (female) in the brain. AcetylCoA half-life was 74 ± 9 h (male) and 71 ± 7 h (female) in the liver; 117 ± 13 h (male) and 158 ± 23 (female) in the brain. These results were in accordance with the corresponding values obtained after intrastriatal infusion of labelled-fosmetpantotenate (CoASH 124 ± 13 h, acetylCoA 117 ± 11 and total CoA 144 ± 17 in male brain).
Conflict of interest statement
Kai Liu and Karsten Baumgaertel are employees of Retrophin, Inc. Daniel Elbaum was employee of Retrophin, Inc. during his work on this project. Laura Orsatti, Edith Monteagudo, Maria Vittoria Orsale, Pamela di Pasquale, Fabrizio Colaceci, Alina Ciammaichella, Ilaria Rossetti and Fabio Bonelli are employees of IRBM working under contract to Retrophin, Inc. Andrea Vecchi was employee of IRBM working under contract to Retrophin, Inc during his work on this project. The authors who are current and former Retrophin employees may have an equity or other financial interest in Retrophin. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

